{ DOWNLOAD AS PDF }
ABOUT AUTHORS:
Ayare P*, Khanvilkar V, Chalak N
Department of Quality Assurance,
Bharati Vidyapeeth’s College of Pharmacy, Navi Mumbai
abprachi@gmail.com
ABSTRACT
Most of the sophisticated analytical techniques like NMR, MS and FT-IR need sample in the extreme pure form which is usually achieved by Preparative column chromatography but it is time consuming. From past few decades, technique called as Flash chromatography is developed which is a modification of Preparative column chromatography. This is an air pressure driven technique comprising of medium and short column chromatography, optimised for rapid separation of organic compounds. Modern flash chromatographic system consists of pre-packed plastic cartridges wherein solvent is pumped through the cartridges to achieve separation. These systems are also linked with detectors and fraction collectors which can even be automated. It is a simple, fast and economic approach to preparative liquid chromatography for purification of chemical species.This review highlights the principle involved, instrumentation, general procedure and advancement in Flash chromatography along with its applications.
REFERENCE ID: PHARMATUTOR-ART-2165
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 5 Received On: 28/02/2014; Accepted On: 25/03/2014; Published On: 01/05/2014 How to cite this article: P Ayare, V Khanvilkar, N Chalak; Flash Chromatography: Area & Applications; PharmaTutor; 2014; 2(5); 89-103 |
INTRODUCTION:
All chromatographic methods –with the exception of TLC- use columns for the separation process. Column chromatography has found its place in many laboratories for preparative purposes as well as for reaction control in organic synthesis. The importance of column chromatography is mainly due to following factors:
Simple packing procedure.
Low operating pressure.
Low expense for instrumentation.
Column chromatography is separated into two categories, depending on how the solvent flows down the column. If the solvent is allowed to flow down the column by gravity, or percolation, it is called Gravity column chromatography. If the solvent is forced down the column by positive air pressure, it is called Flash chromatography.[1] In traditional column chromatography a sample to be purified is placed on the top of a column containing some solid support, often silica gel. The rest of the column is then filled with a solvent (or mixture of solvents) which then runs through the solid support under the force of gravity. The various components to be separated travel through the column at different rates and then can be collected separately as they emerge from the bottom of the column. Unfortunately, the rate at which the solvent percolates through the column is slow. In flash chromatography however air pressure is used to speed up the flow of solvent, dramatically decreasing the time needed to purify the sample, therefore making the column and running the separation could take less than 10-15 minutes.[2]
Flash chromatography is basically an air pressure driven hybrid of medium pressure and shorter column chromatography which has been optimized for particularly rapid separation. Flash chromatography is a technique used to separate mixtures of molecules into their individual constituents, frequently used in the drug discovery process.[1]
Flash chromatography, also known as medium pressure chromatography, was popularized several years ago by Clark Still of Columbia University, as an alternative to slow and often inefficient gravity-fed chromatography. Flash chromatography differs from the conventional technique in two ways:
1. Slightly smaller silica gel particles (250-400 mesh) are used
2. Due to restricted flow of solvent caused by the small gel particles, pressurized gas (10-15 psi) is used to drive the solvent through the column of stationary phase.
The net result is a rapid (“over in a flash”) and high resolution chromatography.[3]
Automated flash chromatography systems include components normally found on more expensive HPLC systems such as a gradient pump, sample injection ports, a UV detector and a fraction collector to collect the eluent. Typically these automated systems separate samples from a few milligrams up to an industrial kg scale and offer much cheaper and quicker solution to doing multiple injections on prep-HPLC system.The software controlling an automated system coordinate the components, allow a user to only collect the factions that contain their target compound and help the user to find the resulting purified material within the fraction collector. The software also saves the resulting chromatograph from the process for archival and/or later recall purposes.[4]
PRINCIPLE OF FLASH CHROMATOGRAPHY:
The principle is that the eluent which is a liquid, under gas pressure (normally nitrogen or compressed air) rapidly pushed through a short glass column. The glass column is packed with an adsorbent of defined particle size withlarge inner diameter. The most used stationary phase is silica gel 40 – 63 μm, but obviously packing with other particle sizes can be used as well. Particles smaller than 25 μm should only be used with very low viscosity mobile phases, because otherwise the flow rate would be very low. Normally gel beds are about 15 cm high with working pressures of 1.5 – 2.0 bars. Originally only unmodified silica was used as the stationary phase, so that only normal phase chromatography was possible. In the meantime, however, and parallel to HPLC, reversed phase materials are used more frequently in flash chromatography.[3]
THEORY:-
Chromatography exploits the differences in partitioning behaviour between a mobile phase and a stationary phase to separate the components in a mixture. Compounds of the mixture interact with the stationary phase based on charge, relative solubility or adsorption. The retention is a measure of the speed at which a substance moves in a chromatographic system. In a continuous development system like HPLC or GC where the compounds are eluted with the eluents, the retention is usually measured as the retention time (rt), the time between the injection and detection. In un-interrupted development system like TLC, the retention is measured as the retention factor (Rf), the run length of the compound divided by the run length of the eluent front. Rf = Distance travelled by the solvent front. [4]
VARIOUS COMPONENT OF FLASH CHROMATOGRAPHY SYSTEM
SORBENT SELECTION:
The basic prerequisite for successful separations is the choice of the proper adsorbent. The most important stationary phase in column chromatography is silica. Silica gel (SiO2) and alumina (Al2O3) are two adsorbents commonly used by the organic chemist for column chromatography. These adsorbents are sold in different mesh sizes, as indicated by a number on the bottle label: “silica gel 60” or “silica gel 230-400” is a couple examples. This number refers to the mesh of the sieve used to size the silica, specifically, the number of holes in the mesh or sieve through which the crude silica particle mixture is passed in the manufacturing process. Adsorbent particle size affects how the solvent flows through the column. Smaller particles (higher mesh values) are used for flash chromatography; larger particles (lower mesh values) are used for gravity chromatography. For example, 70-230 silica gels are used for gravity columns and 230-400 mesh for flash columns. The amount of silica gel depends on the Rf difference of the compounds to be separated, and on the amount of sample. For n grams of sample, you should use 30 to 100 n grams of silica gel. For easier separations, ratios closer to 30: 1 are effective, for difficult separations, more silica gel is often required. However, by using more silica gel, the length of time required for the chromatography is extended. The density of powdered silica gel is about 0.75 g per mL.[1] [2]
These are some adsorbents which are mainly used in flash chromatography:-
· Silica: Slightly acidic medium. Best for ordinary compounds, good separation is achieved.
· Florisil: Mild, neutral medium. 200 mesh can be effective for easy separations. Less than 200 mesh best for purification by filtration. Some compounds stick on florisil, test first.
· Alumina: Basic or neutral medium. Can be effective for easy separations, and purification of amines.
· Reverse phase silica: The most polar compounds elute fastest, the most nonpolar slowest.[5]
The properties of commonly used flash solvents:-
· The compound of interest should have a TLC Rf of ≈0.15 to 0.20 in the solvent system you choose.
· Binary (two component) solvent systems with one solvent having a higher polarity than the other are usually best since they allow for easy adjustment of the average polarity of the eluent.
· The ratio of solvents determines the polarity of the solvent system, and hence the rates of elution of the compounds to be separated.
· Higher polarity of solvent increases rate of elution for all compounds.
· If your Rf is a≈0.2, you will need a volume of solvent ≈5X the volume of the dry silica gel in order to run your column.[4]
Solvent Systems:
Flash column chromatography is usually carried out with a mixture of two solvents, with a polar and a nonpolar component. Occasionally, just one solvent can be use. The only appropriate one component solvent systems (listed from the least polar to the most polar):
1. Hydrocarbons: pentane, petroleum ether, hexanes.
2. Ether and dichloromethane (very similar polarity)
3. Ethyl acetate.
The most common two-component solvent systems (listed from the least polar to the most polar):
4. Ether/Petroleum Ether, Ether/Hexane, and Ether/Pentane: Choice of hydrocarbon component depends upon availability and requirements for boiling range. Pentane is expensive and low-boiling, petroleum ether can be low-boiling, and hexane is readily available.
5. Ethyl Acetate/Hexane: The standard, good for ordinary compounds and best for difficult separations.
6. Methanol/Dichloromethane: For polar compounds.
7. 10 % Ammonia in Methanol Solution/Dichloromethane: Sometimes moves stubborn amines off the baseline.
8.For basic (i.e. nitrogen containing) compounds, it is sometimes useful or necessary to add a small amount of triethylamine or pyridine to the solvent mixture (about 0.1%).
9.For acidic compounds, a small amount of aceticacid is sometimes useful. In these cases, the acetic acid can often be safely rotavaped away by adding portions of toluene and concentrating to a few mL volumes and repeating this several times. As acetic acid boils at a lower bp than toluene, this will remove the acid without exposing the neat compound to it.[1] [5]
Table No.1 :-THE PROPERTIES OF COMMONLY USED FLASH SOLVENTS[2]
|
Solvent |
Density (g/ml) |
Elution Strength |
Solvent Group |
Boiling Point (°C) |
UV Cut-off (nm) |
TLV (ppm) |
|
n-Hexane |
0.66 |
0.01 |
1 |
69 |
195 |
100 |
|
2 2 4-Trimethylpentane |
0.69 |
0.02 |
1 |
99 |
210 |
300 |
|
Cyclohexane |
0.77 |
0.03 |
1 |
81 |
200 |
100 |
|
1 1 2-Trichloromethane |
1.48 |
0.31 |
8 |
61 |
245 |
50 |
|
Toluene |
0.87 |
0.22 |
7 |
110 |
285 |
100 |
|
Dichloromethane |
1.33 |
0.30 |
5 |
40 |
232 |
100 |
|
Ethyl Acetate |
0.90 |
0.45 |
6 |
77 |
256 |
400 |
|
Methyl-t-butyl ether |
0.74 |
0.48 |
2 |
55 |
210 |
40 |
|
Acetone |
0.79 |
0.53 |
6 |
56 |
330 |
750 |
|
Tetrahydrofuran |
0.89 |
0.35 |
4 |
66 |
212 |
200 |
|
Acetonitrile |
0.78 |
0.50 |
6 |
82 |
190 |
40 |
|
Isopropanol |
0.79 |
0.60 |
3 |
82 |
205 |
400 |
|
Ethanol |
0.79 |
0.88 |
3 |
78 |
210 |
1000 |
|
Methanol |
0.79 |
0.70 |
3 |
65 |
205 |
200 |
|
Water |
1.00 |
0.073 |
8 |
100 |
180 |
- |
Column Selection
Select a column that is 10, 20, 40 mm ID based upon preparative requirements. Indeed, Professor Still et al offered this selection given in table below. Single Step Flash Columns (patented) represent an innovative step forward in chromatography. Flash Chromatography is a quick and inexpensive technique for the purification of organic compounds. Thomson flash columns come in a wide variety of sizes ranging from 4g to 300g silica-based for easy scalability of synthetic reactions. Thomson also offers other packing material like Amine and C18 flash columns which enable the end-user to utilize these flash columns for a broad range of reactions.[6]
Table no.2 :-TYPICAL VOLUME OF ELUANT REQUIRED FOR PACKING AND ELUTION [2]
|
Column Diameter (mm) |
Volume of eluent* (ml) |
Sample Load (mg) Rf> 0.2 Rf>0.1 |
Fraction Size (ml) |
|
|
10 |
100 |
100 |
40 |
5 |
|
20 |
200 |
400 |
160 |
10 |
|
30 |
400 |
900 |
360 |
20 |
|
40 |
600 |
1600 |
600 |
30 |
|
50 |
1000 |
2500 |
1000 |
50 |
* Typical Volume required for equilibrium of the column and elution.
Typical Data of Silica gel Column Grade Adsorbents.[7]
Content : <0.02%
Chloride Content : <0.10%
Loss on Drying: <3%
pH(10% suspension) : 7±0.5
Surface Area : 400–600 m2/gm.
Instrumentation of Flash Chromatography[9][10]
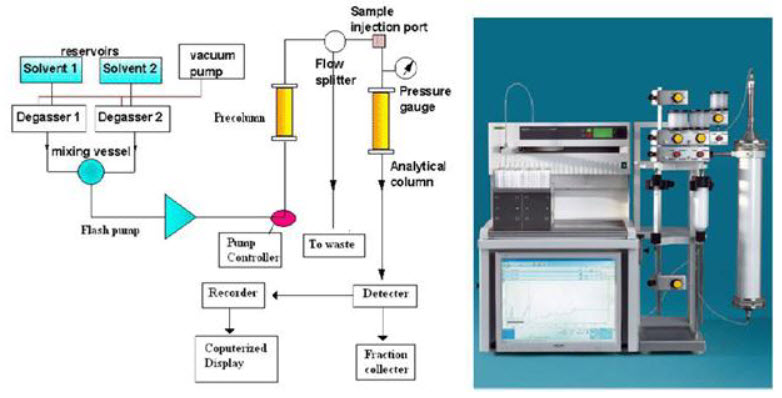
Flash chromatography General consist of following parts
1. Pump Systems.
a) Pump Controller.
b) Vacuum Pump/peristaltic Pump.
2. Sample Injection Systems.
3. Glass Columns, Filling Sets & Column Valves.
4. Precolumns.
5. Fraction Collector.
6. Detectors and Chart Recorders.
7. Computerize LCD Display.
Pump Systems:
Pump Controller
A pressure range up to either 10 bar or 50 bar gives optimum separation results for a broad range of applications. The pump modules can be controlled by three different units. The Pump Controller C610 (for isocratic separation up to 10 bar), the Pump Manager C615 (for isocratic and gradient separation up to 50 bar) and the Control Unit C620.
Pump Controller C-610
The Pump Controller C-610 for one Pump Module C-601 is designed for isocratic separations. The flow rate can be easily adjusted by turning a knob and is indicated by a large illuminated LCD-display. Delivered with a overpressure sensor for maximum safety.
Pump Manager C-615
The Pump Manager C-615 is designed for both isocratic and gradient separations. Fast operation, easy programming and a large graphical display allows a quick and easy set up. Running time, solvent consumption and actual pressure are shown during a separation for maximum optimization. The unit has Input/Outputs for 2 solvent valves and level sensors and includes a pressure sensor and mixing chamber.
Control Unit C-620
The Control Unit C-620 in combination with Sepacore Control provides precise control of the chromatography system. The following components can be connected to the Control Unit C-620: 2 to 4 Pump Modules C-601 or C-605 Up to 2 Fraction Collectors Up to 8 Detectors e. g. UV, RISequential Modules C-623 or C-625 for automatic sequential chromatography on up to 5 columns or cartridges The Control Unit C-620 is included in the Sepacore Control package
Type of pump
Pump Module C-601, 10 bar
The Pump Controller C-610 with a Pump Module C-601 is used for fast isocratic Flash separations. No programming is needed. The system can be run by two buttons and one knob. Pump Module C-601, 10 bar Silent operating 3-piston Pump Module C-601 for flash chromatography. The pump module provides a constant, pulse-free flow from 2.5 to 250 ml/min and ensures reproducible, fast separation at a maximum working pressure of 10 bar/145 psi. For sample sizes of up to 5 g, pre-packed PP cartridges can be used for the quick, safe implementation of normal phase and reversed phase applications.
Pump Module C-605, 50 bar
The Pump Manager C-615 with a Pump Module C-601/C-605 is used for isocratic Flash separations. This combination allows exploration into the features of the Pump Manager C-615 for solvent selection, timed runs and solvent level control. Pump Module C-605, 50 bar Similar to the Pump Module C-601 but with a maximum working pressure of 50 bar/725 psi. Using the Pump Module C-605, fast separation with reversed phase and separations can be performed with sample sizes up to 100 g. Ideal for use with glass and plunger columns and silica gel particle sizes < 40 μm.
Pump Manager C-615
The Pump Manager C-615 with two Pump Modules C-601/C-605 for binary solvent gradients. The efficient solvent mixing under pressure and the pulsation free solvent flow eliminate vapour bubbles and result in maximum separation performance.
Vacuum Pump/peristaltic Pump
Transfer Solvent From Mobile phase Reservoir to Flash Pump.
Sample Injection Systems
Injection systems are designed to facilitate column loading with liquids and low solubility oils and solids. Regardless of the nature or quantity of the material.
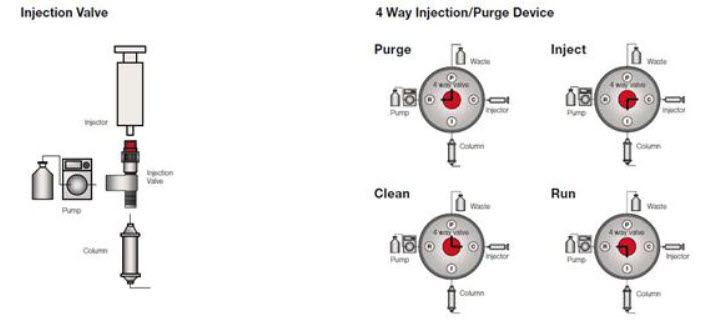
Injection Valve
For the sample injection of 0–5 ml.
Elute Glass Column
Prep Elute Glass Column for use in combination with the Injection Unit for loading dry or barely soluble samples up to either 18 ml or 53 ml. Pressure Range of up to 50 bar or 40 bar.
Sample Chamber 100 ml
Sample Chamber 100 ml for use in combination with the Injection Unit for loading sample volumes of 10 to 100 ml including N2 gas valve (on/off). Glass parts with larger volumes 250 ml, 500 ml and 1000 ml on demand.
Columns
Glass Columns
A wide range of columns offer maximum flexibility for every situation. Depending on the nature and the quantity of the sample offers a series of column types which vary in form, size and performance.
Plastic+Glass Column
Plastic+Glas-coated Glass Columns are available for larger sample amounts and higher pressure applications on a high safety level. The columns are designed for sample amounts from1 – 100 g and pressures up to 50 bar during preparative separations. Easy fixation on a support rod by using the corresponding pivoting clamp.
Plunger Column C-695
Robust, chemically resistant and biocompatible plunger columns are designed for optimum operational performance and safety. Volume changes in soft gel can be equalized and dead volume will be avoided. 1 – 100 g and pressures up to 50 bar during preparative separations. Easy fixation on a support rod by using the corresponding pivoting clamp. An integrated cooling jacket allows separations under constant conditions at a high quality level. Column Length 460 mm.
Precolumns
Precolumn are minimizing dead volumes and enhance the life time of the main column by trapping contaminants. The small Precolumn, fits to Glass Columns of inner diameter of ID 15, 26, 36 and 49 mm. The large Precolumn, fits to Glass Columns of ID 70 and 100 mm inner diameter.
Filling Sets for Glass Columns
Dry Filling Set
The Dry Filling Set is employed for filling glass columns with silica gel using compressed gas. Silica gel in the size range of 25 – 200 μm can be packed with this method.
Slurry Filling Set
The Slurry Filling Set is used for wet filling and conditioning of glass columns with silica gel particles smaller than 25 μm.
Fraction Collector
For simple separations a column, pump and pump controller may be enough. For a greater level of automation with precision, performance and ease of use the Fraction Collector can be incorporated into most setups.
Fraction Collector C-660
The intelligent, height-adjustable Fraction Collector with with dialogue language options for preparative chromatography. The C-660 collects the separated substances according to time, volume or peak.
Detectors and Recorders/Software
For most applications one of the robust UV/Vis detectors would be sufficient for the systems detection needs. Both detectors are delivered in combination with a preparative flow cell. In the absence of adequate UV/Vis absorption, likely for sugars or polymers, a Differential Refractometer (RI Detector) in combination with a UV/Vis detector is the preferred setup.
UV Monitor C-630
Filter Photometer with four standards built in filters at 200 nm, 220 nm, 254 nm and 280 nm. Delivered with built in Deuterium Lamp and a preparative flow cell.
UV Photometer C-635
Spectral Photometer with a wavelength range between 190 nm and 740 nm. Delivered with built in Deuterium Lamp and a preparative flow cell.
Differential Refractometer
Refractive Index detector mostly used in combination with a UV/Vis detector for the analysis of low UV/Vis absorbing substances.Delivered with a preparative cell. For a maximal flow rate of 100 ml/min..
Procedure for Microscale Flash Column Chromatography
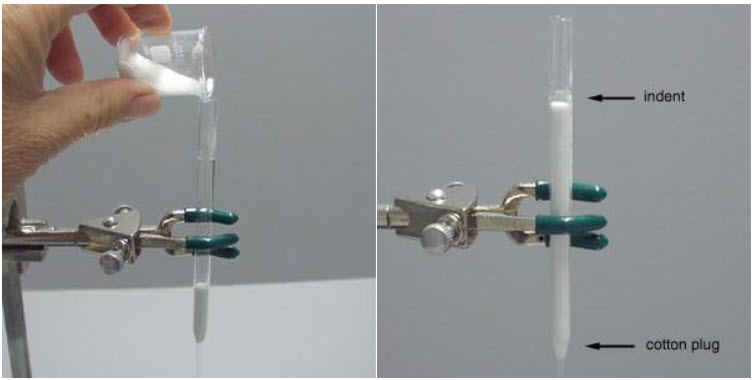
Packing the Column
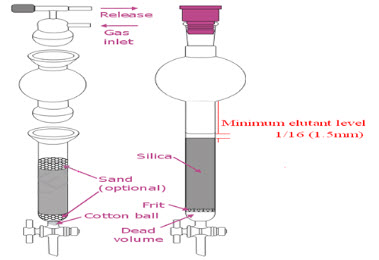
· Obtain a glass column and make sure that it has either a glass frit or a plug of cotton wool directly above the stopcock to prevent the silica gel from escaping from the column through the stopcock.
· Next, put a ~1/2 inch layer of clean sand above the plug of glass wool. Use only as much as is necessary to obtain a flat surface, with the same diameter as that of the body of the column. Make sure the surface is flat. Add dry silica gel adsorbent, 230-400 mesh usually the jar is labelled "for flash chromatography." One way to fill the column is to invert it into the jar of silica gel and scoop it out & then tamps it down before scooping more out.[1]
Another way to fill the column is to pour the gel into the column using a 10 mL beaker. Whichever method we use to fill the column, we must tamp it down on the bench top to pack the silica gel. We can also use a pipette bulb to force air into the column and pack the silica gel. When properly packed, the silica gel fills the column to just below the indent on the pipette. This leaves a space of 4-5 cm on top of the adsorbent for the addition of solvent. Clamp the filled column securely to a ring stand using a small 3-pronged clamp.
Solvating the Silica Gel Column:
· Next, tap gently and evenly the sides of the column with a piece of rubber tubing to settle the silica gel.
· Pour a good amount of elution solvent onto the silica gel. Use pressurized gas to force the solvent through the silica. As we force through a few hundred millilitres, we should see the top part of the silica become more homogeneous. This is because we are forcing out air that was entrapped in the silica gel.
· Continue to flush solvent through the silica gel until the entire silica plug becomes homogeneous in appearance. We may have to recycle the solvent coming through the column onto the top of the column several times before all the silica gel is solvated.[1]
Pre-elute the column
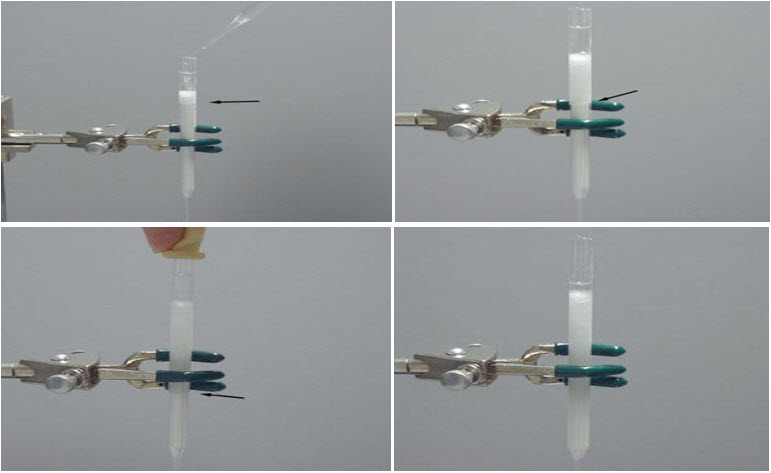
· Add hexanes (or other solvent, as specified by the procedure) to the top of the silica gel.
· The solvent flows slowly down the column; on the column above, it has flowed down to the point marked by the arrow.
· Monitor the solvent level, both as it flows through the silica gel and the level at the top.
· When the bottom solvent level is at the bottom of the column, the pre-elution process is completed and the column is ready to load.[8]
Load the sample onto the silica gel column:
Two different methods are used to load the column: the wet method and the dry method: wet and dry. Below are illustrations of both methods of loading.[8]
Wet Loading Method
In the wet method, the sample to be purified (or separated into components) is dissolved in a small amount of solvent, such as hexanes, acetone, or other solvent. This solution is loaded onto the column. Sometimes the solvent of choice to load the sample onto the column is more polar than the eluting solvents. In this case, if we use the wet method of column loading, it is critical that we only use a few drops of solvent to load the sample. If we use too much solvent, the loading solvent will interfere with the elution and hence the purification or separation of the mixture. In such cases, the dry method of column loading is recommended.
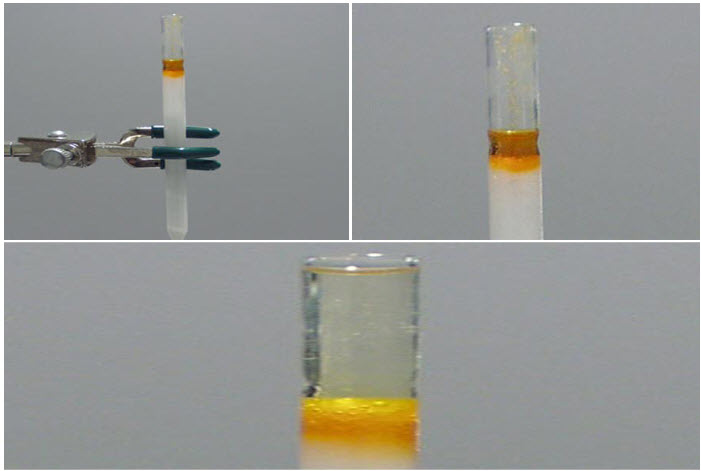
Dry Loading Method:
First dissolve the sample to be analysed in the minimum amount of solvent and add about 100 mg of silica gel. Swirl the mixture until the solvent evaporates and only a dry powder remains. Place the dry powder on a folded piece of weighing paper and transfer it to the top of the prepared column. Add fresh eluting solvent to the top now we are ready to begin the elution process.[8]
Elute the column:
· Add a good part of our elution solvent to the column.
· Apply pressure to force the solvent through the column by pressing on the top of the Pasteur pipette with a pipette bulb. Only force the solvent to the very top of the silica: do not let the silica go dry. Add fresh solvent as necessary.
· The pressure should be the minimum necessary to keep a steady stream coming out of the column. Figure below shows the colored compound as it moves through the column after successive applications of the pipette bulb process.
· The collection beaker is changed as soon as the colored compound begins to elute. The process is complicated if the compound is not colored. In such experiments, equal sized fractions are collected sequentially and carefully labelled for later analysis.[8]

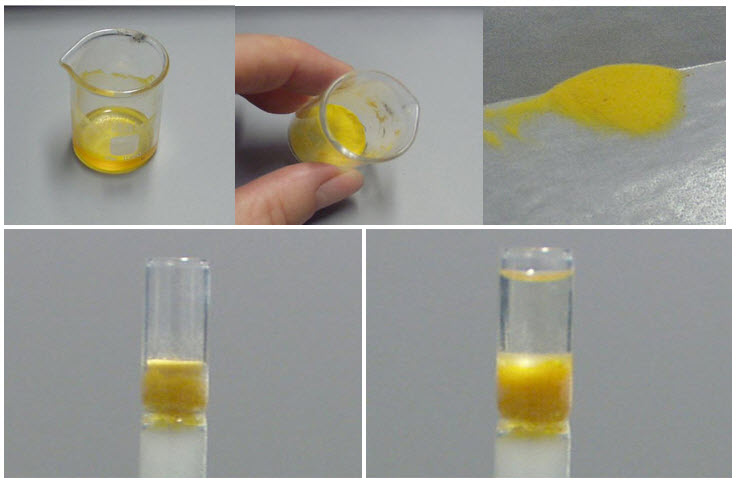
Analyze the fractions:
· If the fractions are colored, we can simply combine like-colored fractions, although TLC before combination is usually advisable.
· If the fractions are not colored, they are analyzed by TLC (usually). Once the composition of each fraction is known, the fractions containing the desired compound(s) are combined.[8]
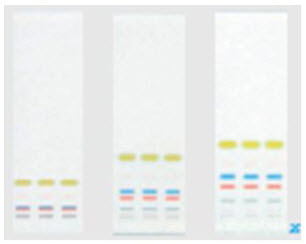
Cleaning the Column:
· Flush all the remaining solvent out of the column using pressurized gas. Flowing air through the column for ~2 hours will give dry, free flowing silica gel.
· Pour out the contents of the column into the silica waste container.In most cases, washing the column with water and acetone is sufficient.
· If necessary, a small amount of liquid soap can be used.When all liquid solvent has been removed from the reservoir, remove the last remnants of solvent by applying a vacuum (from aspirator) to the bottom of the column. Try to avoid scratching the columns with abrasive brushes or soaps.[1] [5]
MODERN FLASH CHROMATOGRAPHIC TECHNIQUES
Pre-packed plastic cartridges[15]
In the modern Flash Chromatography system, the glass columns are replaced with pre-packed plastic cartridges which are much safer and also more reproducible.Solvent is pumped through the cartridge, which is much quicker and more reproducible. Systems may also be linked with detectors and fraction collectors providing automation.The introduction of gradient pumps means quicker separations, less solvent usage and greater flexibility.
Column characteristics:
1. Disposable plastic cartridges –save time and increase reproducibility
2. Cartridges of different size -easy scale-up
3. Solid sample module and injection valve - easy sample loading
4. Pressure up to 100 psi - fast separation
5. Narrow particle distribution - Low backpressure and higher efficiency

Advanced Detection Techniques for Flash Chromatography[11]
UV detection is the traditional method used in Flash chromatography to monitor and fractionate peaks during the purification process. There are few detection options available in Flash chromatography for compounds that lack chromophores, and thus cannot be detected by UV. These invisible compounds may not be detected with UV due to the absence of UV chromophores, or their absorbance may be “lost” in that of the solvents used in Flash chromatography. In other cases, the compounds’ absorption spectrum may be unknown and or detection was at a sub-optimal wavelength. Additionally, the UV absorption of the necessary mobile phase may interfere with the λ-max of the compound in question. In other cases, the absorption spectrum of the compound of interest or co-eluting impurities may not be known and therefore not detected. These advanced detection techniques allow users to easily fractionate compounds without the need for follow-up TLC and subsequent staining to determine where the purified compound eluted.
Evaporative Light Scattering Detection (ELSD) has long been used for High Performance Liquid Chromatography, but has only recently been employed for Flash chromatography. All-Wavelength Collection allows the collection of compound with unknown absorbance or collection in the presence of interfering solvent absorbance.[11]
Green Flash Chromatography[16]
Green Flash Chromatography is the ultimate flash chromatographic technologythat achieves most efficient sample purification.The sample run is always carried out with minimum eluting volume.It minimizes run time and solvent use while achieving a good separation.It is Eco-friendly. Optimum method will be developed automatically based on the true theory of the flash chromatography, with the simple input of the TLC results, which allows easy sample purification.
Features of Green Flash Chromatography
- Optimal parameters for flow rate, run time, fraction volume, etc. will be calculated and set automatically upon selecting a column on “Green Flash” software. The default parameters will be shown in System Setting window.
- Software provides the maximum sample load information for the selected column.
- State-Of-The-Art Software Based On True Theory of Chromatography.
- Sample Eluting Position and Resolution Can Be Fully Controlled for Systems.
- Automatic Method Setup for Reverse Phase Chromatography.
- Parallel Detection of UV Detector and RI Detector or ELSD.
FLASH CROMATOGRAPHYWITH TLC IMAGE READER.[14]
The system is equipped with a built-in UV light source and a camera. By shooting the TLC plate and clicking the target compound on the TLC plate, the Rf value of the target compound will be calculated and the optimized chromatography method will be developed automatically. The TLC plate is displayed on the screen during run. Compound spots on the TLC plate and the compound peaks both are displayed on the screen. Both the photographic image of the TLC plate and the purification are saved as a data file. Click the target compound and the nearest impurity on the TLC plate, and the maximum sample load for each column will be automatically calculated. Thus, enabling the chemists to choose the best suited column for their sample.
Advantages[14]
* Fast and economic methods for the synthesis laboratory.
* Ideal for the separation of compounds up to gram quantities.
* No expensive equipment required.
* In an ideal way transfers results from TLC to CLC.
* Automated changes between normal phase and reversed phase chromatography.
APPLICATIONS OF FLASH CHROMATOGRAPHY:-[12] [13]
Natural Products/Nutraceuticals Application:
1. Separation and Isolation of α-Santalol and β-Santalol from Sandalwood Extraction
2 .Isolation and Purification of Chromophoric and Nonchromophoric Compounds in Giant Knotweed Rhizome.
3.Isolation and Purification of Flavonoids from Ginkgo Biloba Leaves Extract.
4.Isolation and Purification of Ginsenosides from Red Panax Ginseng Extract.
5.Isolation and Purification of Catechins from Green Tea Extract .
6.In Purification of GallaChinensis.
7.Purification of Ferulic Acid in RhizomaChuanxiong Extract.
Carbohydrate Application:
1. Purification of Conjugated Quercetin and Rutinose.
2. Impurity Isolation of Valproic Acid from CyclodextrinvDuring Encapsulation.
3. Isolation ofAminosugar and Acarbose .
4. Flavanone Glycoside Purification.
5. Isolation of Aminoglycoside Antibiotics.
Lipids Application:
1. Purification of Fatty Acid Methyl Esters (FAMEs).
2. Purification of a Mixture of Glycerides, Mono-, Di-, and Tristearin.
3. Purification of Sterols.
Pharmaceutical/Small Molecules Application:
1. Bile Acid Purification During Lead Generation in Drug Discovery.
2. In Impurity Isolation During Drug Purification.
3. Mestranol Purification During Chemical Synthesis.
4 .In Anti-malarial Drug Purification in Drug Discovery.
Conclusion:
Purification of drug is an important step in any branch of research.Preparative chromatography is used to separate the components of a mixture for more advanced use and is thus a form of purification. Flash Chromatography can be alternative to preparative HPLC as it saves time and solvent. Extrapolation of TLC results on preparative scale can be achieved by Flash chromatography. Modern Flash chromatography with disposable cartridges and advanced detection techniques is applicable to a wide range of compounds.
REFERENCES:
1.Still WC. Kahn M., Mitra A, Flash chromatography, J.Org.Chem. 43(14)1978; 2923-2925.
2.A. B. Roge*, S. N. Firke, R. M. Kawade, S. K. Sarje, and S. M. Vadvalkar,BRIEF REVIEW ON: FLASH CHROMATOGRAPHY,IJPSR (2011), Vol. 2, Issue 8,1930-1937.
3.William CSand Hill DC. General methods for flash chromatography using disposable column. Mol. Divers, 13(2), 2009, 247-252.
4.HetalChaudhari*, FalguniChaudhari, Madhavi Patel, P.K. Pradhan, U.M. Upadhyay,A REVIEW ON A FLASH CHROMATOGRAPHY,International Journal of Pharmaceutical Development & Technology,2 (2), 2012, 80-84.
5. chem.rochester.edu/how to flash.html
6. saiadsorbants.com
7. sorbeadindia.com
8. orgchem.colorado.edu.
9. Buchi Preparative Chromatography.
10.Dane Ganesh D*, Raka KC, Honde BS, Bhawal GS, Tajane PJ, REVIEW ON FLASH CHROMATOGRAPHY,Vol 3,Issue 1, 2013 ,45-49.
11.jsilver@teledyne.com
12.Dewick, P.; John Wiley & Sons, Inc., Hoboken, Medicinal natural products; a biosynthetic approach, 3rd edition ,New Jersey, 2009.
13. discoverysciences.com
14. pretech.nu/products
15. sigmaaldrich.com
16. yamazenusa.com
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









