About Author: Imran A. Kayyum. Tadwee1*, Sadhana Shahi1, Mahesh Thube1
Government College of Pharmacy,
Aurangabad, Maharashtra, India
Reference ID: PHARMATUTOR-ART-1051
Abstract
The object of this work is to formulate & observe the effect of HPMC K15 polymer on various parameters of evaluation of carbamazepine nasal mucoadhesive microspheres. The microspheres are prepared by spray drying technique with different formulation composition of HPMC K15. The microspheres obtained are meant for the evaluation for its production yield, particle size, swelling ability, Drug content, encapsulation efficiency and Invitro drug release study. IR, TLC study is performed for drug Polymer interaction study of the optimized batch. Morphology is studied be SEM. The results obtain shows that the release rate of the drug decreases and particle size, production yield, swelling index, encapsulation efficiency, mucoadhesion is increases with increase in drug: polymer ratio. TLC study shows no interaction of Carbamazepine and HPMC. SEM study reveals the encapsulation of drug in the HPMC K15 Polymer. The data obtained after Invitro release study fitted to PCP disso software for mechanism of release & kinetic model. The data also treated with design expert software v 7.1 it shows the model is significant. Overall it shows that the polymer HPMC does not posses any interaction with the carbamazepine and showing controlled drug release behavior.
[adsense:336x280:8701650588]
Background
The drug carbamazepine is BCS class II drug having very low solubility and various issues in the solubility in GI track. Various approaches have been made to improve the solubility of drug with the different polymers like Chitosan, Gellan Gum etc. by using HPMC K4 immediate release formulation is also prepared and showed the promising results for the drug delivery of carbamazepine1. This approach is done to observe the effect of HPMC K15 on the rate of drug release and other parameters of formulation composition describe in table 1
Introduction
The Hydroxypropyl methylcellulose is a methylcellulose modified with a small amount of propylene glycol ether groups attached to the anhydroglucose of the cellulose2. The nasal drug delivery finds the alternative way of targeting the drug to CNS with the advantage of its high vasculerisation3, and faster onset of action. The carbamazepine is an antiepileptic drug which is used effectively in epileptic seizures. The HPMC is having the ability to swell and perform the modified release of the drug from it. By incorporating the drug in the HPMC K15 polymer the drug release effect might have different behavior. By this study it is aim to observe the behavior of drug from polymer and its evaluation for effective nasal drug delivery system.
Materials
Carbamazepine is obtained as a gift sample from CTX life science Surat India. HPMC K15 is procured as gift sample from Colorcon Goa. Methanol and Dichloromethane were procured from Deepa Chemical (Aurangabad, India). Deionized water was used for all of the experiments
Preparation of Drug Loaded HPMC Microspheres:
The HPMC K15 based microspheres are prepared by spray drying technique with formulation composition stated in table 1. The operating parameter of spray dryer (JISL, India) was inlet temperature 70° C outlet temperature 55° C, feed pump 9/10 ml /min, aspirator speed 40–50% 3.
Table 1 Formulation Composition
|
Formulation code |
Drug: polymer ratio |
Drug (mg) |
Polymer (mg) |
Dichloromethane (ml) |
|
M1 |
1:1 |
200 |
200 |
60 |
|
M2 |
1:2 |
200 |
400 |
80 |
|
M3 |
1:3 |
200 |
600 |
100 |
|
M4 |
1:4 |
200 |
800 |
120 |
|
M5 |
1:5 |
200 |
1000 |
150 |
Characterization:
Production Yield:
The microspheres obtained were evaluated for production yield by formula Practical yield upon theoretical yield into hundred.
Particle size:
The particle size of the microspheres is observed with optical microscope (Olympus) equipped with software magnus pro 2.1 the image is clicked and saved.
[adsense:336x280:8701650588]
Swelling Index: As the HPMC having ability to swell the swelling index for HPMC K15 prepared microsphere in physiological media was determined by allowing them to swell to their equilibrium. Accurately weighed amount of microspheres (20 mg) were placed on Millipore filter (NY 11 0.22 mm) using a Franz type diffusion cell (12.5 ml) with phosphate buffer (pH 6.6) and kept for 10 min. The degree of swelling is calculated by initial weight of microsphere minus Final weight of microspheres after swelling upon Final weight of microspheres after swelling 4.
Encapsulation Efficiency & Drug Content: The weighed amount of microspheres were dissolved in distilled water and kept overnight. The drug content was measured spectrophotometrically at 285 nm for carbamazepine 5
In Vitro Drug Release Study: To observe the effect of HPMC K15 on release of Carbamazepine from microsphere the six formulations were studied for invitro drug release study. The release study performed by using nasal diffusion cell using dialysis membrane having 135000 MW. The chamber has total capacity of 60 ml and a flanged top of 3 mm, the lid has three openings, one each for sampling, thermometer and a donor tube chamber. The donor chamber was a 10 cm long tube with internal diameter of 1.13 cm. The donor chamber tube was placed in such a way that it just touched the diffusion medium in the receptor chamber 6. The receptor compartment contained phosphate buffer solution pH 6.6 that was within the pH range in nasal cavity and maintained at 37°C±1°C. 10 mg of microspheres were placed on the donor side and samples were withdrawn of 30 min & 1hr interval. The data obtained were fitted to PCP disso software. % drug release.13
Thin Layer Chromatography:
Drug-excipients interaction study was done by thin layer chromatography. Precoated silica gel aluminium plate 60 F254 (10 cm × 20 cm, layer thickness 0.2 mm, E-merck, Darmstad, Germany) was used as stationary phase. These plates were prewashed with methanol and used for study. The mobile phase consisted of benzyl: methanol 9:1 v/v). Iodine vapors were used as visualizing agent. Physical mixtures of drug and excipients were filled in the washed ampoules then sealed. The sealed ampoules were kept at 37 ± 0.50C for 28 days in stability chamber. Then Rf value of plain drug and mixtures were determined
Surface Electron Microscopy: The morphology of the optimized formulations (M1) was studied using a scanning electron microscope (JSM 6390 India) operated at an accelerating voltage of 20 kV.
Histopathological Study: The HPMC K15 loaded Microsphere were studied for Histopathological evaluation to check whether the formulation is having destructive effect on nasal mucosa or no for this sheeps nasal mucosa is obtained from local abbaitor house and used within 1 hr.
Drug Release Mechanism: The release data were fitted to the PCP disso version 2.08 software. The statistical treatment of data is done by Design Expert software version 7.1.6 and for study and analysis the release kinetics of optimized formulation (M1), data obtained from in vitro drug release studies were plotted in various kinetic models such as zero order as cumulative amount of drug released Vs time, first order as log cumulative percentage of drug remaining Vs time and Higuchi’s model as cumulative percentage of drug released Vs square root of time.
Statistical Treatment: The data obtained for the selected variable i.e. HPMC K15 were treated using Stat Ease Design Expert 7.1.4 software and analyzed statistically using analysis of variance (ANOVA).
Result and Discussion:
Production Yield:
The microspheres obtained were evaluated for production yield the yield obtained is shown in the table 2
Particle Size:
The particle size of the microspheres found in the range of the required for nasal delivery (Figure 1) & it is reported in table 2
Figure 1: Particle Size of The Microsphere.
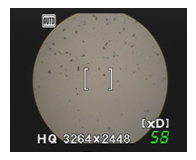
Swelling Index: As the concentration of HPMC increases the swelling of microspheres is increased. The swelling ability of the HPMC based microspheres is stated in the table 2
Encapsulation Efficiency & Drug Content: Maximum drug loading is found with the optimized formulation M1 and incorporation efficiency with M6 because of higher HPMC content. The result are shown in the table 2
Table 2: Characterization of Hpmc Based Formulation
|
Formulation code |
Drug loading (%) * |
Incorporation efficiency (%) # |
Swelling index (%) ¥ |
Mucoadhesion (%) € |
Production yield (%) * |
Average particle size (µm) # |
|
M1 |
41.91±1.77 |
83.82±1.44 |
0.3507 ±1.56 |
81.07 ± 1.58 |
43.75 ± 2.04 |
25.54±2.78 |
|
M2 |
29.30±0.59 |
87.98±1.63 |
0.4130 ±1.78 |
83.82 ± 2.21 |
48.16 ± 2.78 |
29.96±2.32 |
|
M3 |
21.46±1.47 |
89.84±1.87 |
0.4727 ±0.89 |
86.06 ± 2.09 |
49.75 ± 1.09 |
36.77±3.41 |
|
M4 |
18.10±1.55 |
90.502.03 |
0.5304 ±0.55 |
88.61 ± 1.17 |
52.10 ± 0.69 |
38.47±2.15 |
|
M5 |
15.20±2.21 |
91.23±1.44 |
0.5767 ±0.74 |
90.26 ± 0.49 |
54.50 ± 0.89 |
43.37±3.09 |
|
M6 |
13.20±1.04 |
92.43±0.73 |
0.6184 ±0.98 |
91.81 ± 1.62 |
56.14 ±1.73 |
49.55±1.56 |
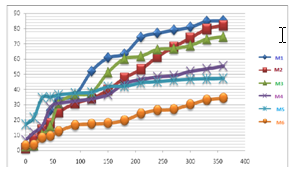
In Vitro Drug Release Study: In vitro drug release shows the modified release behavior of the drug from HPMC based microspheres. As the concentration of the HPMC K15 concentration is increased the drug release is decreased and shows the control release type of behavior the formulation (Figure 2).
Figure 2 Release Pattern of Drug From Hpmc Polymer Microspheres
Thin Layer Chromatography:
Drug excipients study was carried out by using TLC. Results are shown in following table no 3.
Table 3: Rf Values of Drug And Drug Loaded Microspheres
|
Ingredients |
Rf Value |
|
Carbamazepine |
0.68 |
|
Carbamazepine + HPMC K15 M |
0.67 |
Standard Rf value of Carbamazepine is 0.69 which was reported in literature. The results showed that Rf value of carbamazepine was 0.68 and other mixture of drug and polymers have no any major difference compare to standard Rf value of drug. So from TLC study it concludes that there was no interaction between drug and polymers.
Surface Electron Microscopy:
Morphology of HPMC K15 Polymer loaded with drug is observed for optimized formulation by surface electron microscopy and found the spheres of drug loaded polymer with slightly rough surface (Figure 3)
Fig. 3 SEM Graph of Drug Loaded Hpmc Microspheres

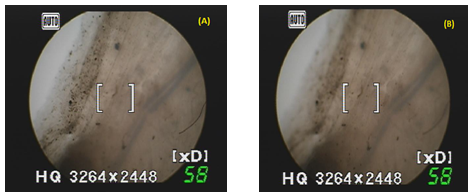
Histopathological Study: It is necessary to examine histological changes in nasal mucosa caused by formulations. The histological study was performed staining the normal mucosa with hematoxylin solution (Figure 4A) and treated with the formulation F1 for 8hr it was found that there was no change in mucosal structure (Figure 4B).
Figure 4: Histopathological Images of Microspheres Applied on Nasal Mucosa
Drug release mechanism: The result obtained is treated with the PCP disso software for drug release mechanism the result obtained is shown in the table 4
Table 4: Result of Optimized Formulation Treated For Drug Release Mechanism
|
Formulation Code |
Kinetic models
|
|
|
|
||||
|
|
Zero order |
First order |
Higuchi matrix |
Korsmeyer peppa’s |
Best fit model |
k |
n |
Mechanism |
|
M1 |
0.951 |
0.978 |
0.915 |
0.995 |
Korsmeyer peppa’s |
2.429 |
0.724 |
Non-fickian diffusion |
Statistical Treatment: The linear model for release was found to be significant. The increase in the concentration of HPMC K15 resulted in decrease in the release of carbamazepine. The Model Fvalue for HPMC K15 is 165.17 28 implies both models are significant (Table 5&6)
Table 5: Statistical Treatment of Data
|
HPMC K15 |
||||||
|
Model HPMC K15 |
3068.056 |
1 |
3068.056 |
165.1748 |
< 0.0001 |
Significant |
|
Residual |
92.87302 |
5 |
18.5746 |
|
|
|
|
Lack of Fit |
92.24802 |
3 |
30.74934 |
98.39788 |
0.0101 |
Significant |
|
Pure Error |
0.625 |
2 |
0.3125 |
|
|
|
|
Cor Total |
3160.929 |
6 |
|
|
|
|
Table 6: R-Squared and Final Equation In Terms Of Coded Factor Values By Design Expert Software
|
Polymer |
Std. deviation |
Mean |
C.V (%) |
R2 |
Adjusted R2 |
Predicted R2 |
Adequate precision |
Final equation in terms of coded factor |
Final equation in terms of actual factor |
|
HPMC K15 |
4.309 |
60.214 |
7.157 |
0.970 |
0.964 |
0.954 |
22.66 |
60.21 -26.11 |
96.76-0.05 |
Conclusion:
The HPMC based microspheres is prepared and evaluated with consideration of nasal delivery of Carbamazepine. The basic aim was to study the behavior of HPMC with the Carbamazepine on the effective nasal delivery. For this the formulation is evaluated for various parameters and found the promising result for effective drug delivery for nasal administration. The particle size of the microsphere found in the range for nasal delivery. Mucoadhesion, swelling ability, production yield, increased with increased in drug: polymer ration. Spray drying is a suitable technique for preparation of mucoadhesive microspheres of carbamazepine based on HPMC. On the basis of all preliminary results, this study concludes that the microspheres based on a K15M polymer may be considered as a promising nasal delivery system for the administration of carbamazepine. The designed drug and polymer system also holds promise to further study i.e. in vivo studies leading to IVIVC for commercialization.
References:
1. Tadwee Imran AK, Shahi SR, Thube MW, Shaikh AM, Tribhuwan SD, Mahajan HS Spray Dried Nasal Mucoadhesive Microspheres of Carbamazepine: Preparation and In-vitro/Ex-vivo Evaluation: Journal of scientia publication res pharmaceutica 2011 I Vol. 2 I Issue 1 I 23-32
2. https://www.greatvistachemicals.com/industrial_and_specialty_chemicals/hydroxypropyl_methylcellulose.html
3. 1 Chein YW, Su KS, Chang SF. Nasal systemic drug delivery. Marcel Dekker Inc: New York; 1989.
4. Snehal A. Jain a; Dheeraj S. Chauk a; Hitendra S. Mahajan a; Avinash R. Tekade; Surendra G. Gattani a Formulation and evaluation of nasal mucoadhesive microspheres of Sumatriptan succinate Journal of Microencapsulation, 2009, 1–11.
5. Patel JK, Patel RP, Amin AF, Patel MM. Formulation and evaluation of mucoadhesive glipizide microspheres. AAPS Pharm Sci Tech 2005;6:E 49-55
6. Fu-De C, Ming-Shi Y, Ben-Gang Y, Yu-Ling F, Liang W, Peng Y, et al. Preparation of sustained-release nitrendipine microspheres with eudragit RS and aerosil using quasi-emulsion solvent diffusion method. Int J Pharm. 2003; 259:103–13.
7. Bhise SB, Rajkumar M. Controlled nasal delivery of ondansetron HCl. Indian J Pharm Educ Res. 2007;41:229–35.
8. Hameed MD, Kellaway IW. Preparation and in vitro characterization of mucoadhesive polymeric microspheres as intra-nasal delivery systems. Eur J Pharm Biopharm. 1997; 44:53–60.
9. Michael IU, Norbert V, Renaat K. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J Pharm Pharmacol. 2001; 53:3–22.
10. Ugwoke M, Agu R, Verbeke N, Kinget R. Nasal mucoadhesive drug delivery: background, applications, trends and future perspectives. Adv Drug Del Rev. 2005; 57:1640–65.
11. Benita, S. Microencapsulation: Methods and Industrial Applications. 10th Ed. Vol 73. New York: Marcel Dekker Inc, 31- 7.
12. Illum, L, Jorgensoen, H, Bisgaard, H, Krogsgaard, O, Rossing, N.(2001) Bioadhesive microspheres as a potential nasal drug delivery system. Int. J. Pharm., 222, 109-119.
13. Pisal S, Shelke V, Mahadik K, Kadam S. Effect of organogel components on in vitro nasal delivery of propranolol hydrochloride. AAPS Pharm Sci Tech 2004;5:63
14. Dandagi, PM, Mastiholimath, VS, Gadad, AP, Iliger, SR. (2007) Mucoadhesive microspeheres of Propranolol Hydrochloride for Nasal Delivery. Indian Journal of Pharmaceutical sciences, 69, 402- 7.









