ABOUT AUTHORS:
Piyush Tripathi*, Rahul Sharma, Shanti Bhushan Mishra
Kota College of Pharmacy, Ranpur,
Kota-325003 (Rajasthan) India.
*piyushtripathi1992@rediffmail.com
ABSTRACT
A biomarker can be a substance that is introduced into an organism as a means to examine organ function or other aspects of health. A biomarker is a parameter that can be used to measure the progress of disease or the effects of treatment. In the treatment of diseases especially “Cancer”, there is a shift from the traditional clinical practices (drugs with low toxicity) to novel approaches. Currently, effective treatment is available for only a small percentage of cancer patients.These novel approaches are intended to identify individualized patient benefits of therapies, minimize the risk of toxicity and reduce the cost of treatment. The biggest challenge for researchers and clinicians today is, to decide on which type of biomarker to use across the wide spectrum of disease processes. In cancer, genomic studies are valuable because every cancer cell shows some degree of genetic damage. Biomarkers have the ability to greatly enhance cancer detection and the drug development process. In addition, biomarkers will enable physicians to develop individualized treatment plans for their cancer patients; thus allowing doctors to tailor drugs specific to their patient's tumor type.
REFERENCE ID: PHARMATUTOR-ART-1834
BIOMARKERS DEFINED
Ideally, different organizations and publications would agree on the definition of a biomarker. However, defining biomarkers is not straightforward because the term is used in a number of different disciplines and the types of biological measures that are considered biomarkers have expanded over time.
According to the NIH (National Institutes of Health), a biological marker or biomarker is defined as:
“A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention[1].”
|
Definitions of Biomarkers |
|
|
Source |
Definition |
|
National Cancer Institute |
A biological molecule found in blood, other body fluids, or tissues that is a sign of a normal or abnormal process, or of a condition or disease. A biomarker may be used to see how well the body responds to a treatment for a disease or condition. Also called molecular marker and signature molecule. |
|
MedicineNet dictionary |
A biochemical feature or facet that can be used to measure the progress of disease or the effects of treatment |
A biomarker can be a substance that is introduced into an organism as a means to examine organ function or other aspects of health. A biomarker is a parameter that can be used to measure the progress of disease or the effects of treatment.
An ideal biomarker should be safe and easy to measure.
• The cost of follow-up tests should be relatively low, there should be proven treatment to modify the biomarker.
• It should be consistent across genders and ethnic groups.
HISTORY OF BIOMARKERS
Probably the most famous biomarker in recent drug development history is the HER-2 gene and receptor, discovered in the mid 1980’s. Between 20–30% of breast cancer patients show an over-expression of the HER-2 receptor on their cancer cells. Although this biomarker indicates a higher risk of adverse outcomes, it also gave clinicians a new target for novel therapies. The antibody trastuzumab (Heretic) was developed to target HER-2 receptors in these over expressing patients, and successfully reduces the proliferation of cancer cells in many of these women [2,3].
Biomarkers based on drug development can be described as Diagnostic biomarkers provide the means to define a population with a specific disease. Prognostic biomarkers correlate with outcomes. For example, over expression of Her-2/neu in breast cancer or EGFR expression in colorectal cancer indicates poor prognoses. Such prognostic markers are frequently the basis for establishing inclusion criteria for a clinical trial or for defining a patient population. It is also know as cancer biomarkers, and biomarkers for monitoring the clinical response to an intervention [4].
BIOMARKERS AN EMERGING TOOL
Biomarkers are useful throughout the drug discovery and development process. In the past, biomarkers have tended to appear in drug development programs as opportunists
– taking advantage of spare samples and leftover money in the budget – often resulting in incomplete or inadequate data. However, they are now becoming more and more
integrated into all stages of the development process, ranging from:
Target discovery
Evaluation of drug activity
Understanding mechanisms of action
Toxicity and safety evaluation
Internal decision making
Clinical study design
Diagnostic tools
Understanding disease processes
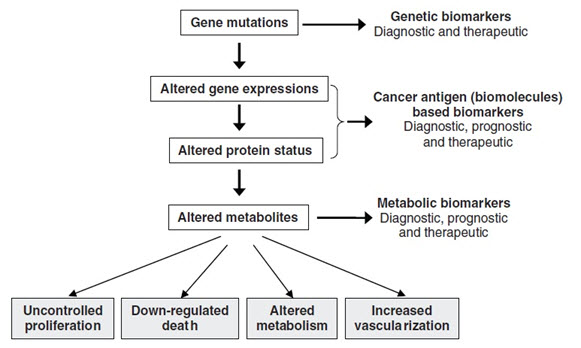
The process of carcinogenesis, showing opportunities of identifying biomarkers
Biomarkers can be of varying types, such as physiological, physical, anatomical and histological (tissue biopsy specimens). Perhaps the most relevant type for early phase clinical research is biochemical biomarkers, derived from bodily fluids that are easily available to the early phase researchers. The use of pharmacodynamic markers in drug development, typically blood based biomarkers that are influenced by drugs, is a fresh approach [5].
A Short Overview on Worldwide Cancer Statistics :-
Cancer rates could further increase by 50% to 15 million new cases in the year 2020, according to the World Cancer Report, the most comprehensive global examination. The World Cancer Report tells us that cancer rates are set to increase at an alarming rate globally. We can make a difference by taking action today.
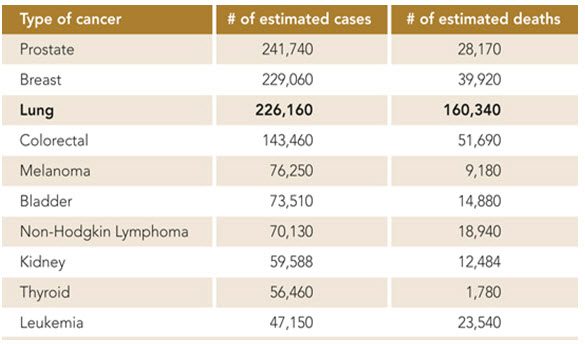
Information from 2011 Cancer Program Annual Report, Wake Forest Baptist Health
This chart shows that lung cancer is the third most common cancer in the United States in 2012, but is by far the leading cause of cancer deaths.
BIOMARKERS - DISCOVERY & DEVELOPMENT–
The need to evaluate novel biomarkers in parallel with novel drug targets is exemplified by disorders that have a long presymptomatic period, such as Alzheimer’s and Parkinson’s disease [6-8]. This parallel approach should reduce the attrition of drugs in development.
Biomarker development is a multi-stage process in which validated targets or drugs can be used to identify biomarkers in the development phase. Also, in the preclinical phase, biomarkers can be used to monitor pharmacological and toxicological effects in in vitro or in vivo model systems. A sequential set of biomarkers could facilitate drug development more effectively than a single assay.
In fact, the current increased rate of novel target identification can be attributed to an increasing understanding of mechanisms of disease and toxicity as well as effects of therapeutic intervention brought about by the ‘omics’ technologies.
The field of biomarker discovery and development is complex and broad. This review will not attempt to present a comprehensive assessment of all aspects relating to this topic, but rather will focus on the second phase of biomarker development, especially “cancer” based.
The biggest challenge for researchers and clinicians today is, to decide on which type of biomarker to use across the wide spectrum of disease processes. In cancer, genomic studies are valuable because every cancer cell shows some degree of genetic damage.
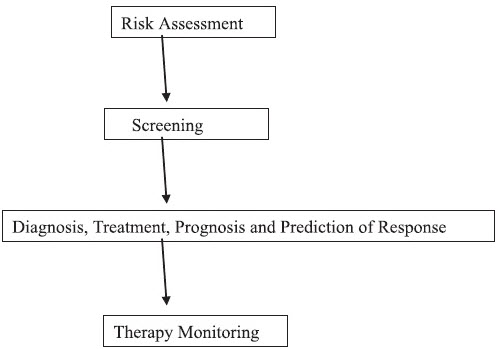
CANCER BIOMARKERS
A handful of cancer biomarkers are currently used routinely for population screening, disease diagnosis, prognosis, monitoring of therapy, and prediction of therapeutic response.Even prostate-specific antigen (PSA), which has been approved for population screening by the Food and Drug Administration, is not universally accepted for this application. The reasons for biomarker failure in population screening settings are multiple and fall outside the scope of this review. It will suffice to mention that poor specificity leads to many false-positive results [9]. It can be concluded that none of the individual biomarkers currently at hand can fulfill the requirements of population screening for cancer.
Biomarkers are clinically recommended mainly for monitoring the effectiveness of therapeutic interventions. Some biomarkers are also invaluable tools for early diagnosis of cancer relapse, which may trigger additional treatments before the appearance of clinical symptoms. With current cancer biomarkers, much is left to be desired in terms of clinical applicability. We need new cancer biomarkers that will further enhance ourability to diagnose, prognosis, and predict therapeutic response in many types of cancer. Because biomarkers can be analyzed relatively noninvasively and economically, it is worth investing in discovering more biomarkers in the future. The completion of the Human Genome Project has raised expectations that the knowledge of all genes and proteins will lead to the identification of many candidate biomarkers for cancer and other diseases. This prediction still needs to be realized. We are now bound to discover biomarkers that are less sensitive or specific but that could be used in panels, in combination with powerful bioinformatics tools (such as artificial neural networks logistic regression etc.), to devise diagnostic algorithms with improved sensitivity and specificity[10,11].
GENERAL STRATEGIES FOR DISCOVERING NEW CANCER BIOMARKERS
Most of the currently used cancer biomarkers were discovered following development of novel analytical techniques, such as immunological assays and the monoclonal antibody technology. It was then found that these molecules were elevated in biological fluids from cancer patients in comparison to normal subjects. Many cancer biomarkers were discovered by immunizing animals with extracts from tumors or cancer cell lines, and then screening for monoclonal antibodies that recognize “cancer-associated” antigens. More recently, and with the completion of the Human Genome Project, many researchers hypothesized that the best cancer biomarkers will likely be secreted proteins [12]; about 20–25% of all cell proteins are secreted. However, this is not an absolute requirement because a number of classical cancer biomarkers (e.g. CEA, Her2-neu) are cell membrane-bound, but their extracellular domains are shed into the circulation.
Other groups, including our own, are using bioinformatics, such as digital differential display and in silico Northern blotting, to compare gene expression between normal and cancerous tissues to identify over expressed genes [13]. Although one of the prevailing hypotheses in new biomarker discovery is that the most promising biomarkers should be over expressed proteins, this is not generally true for some of the best known cancer biomarkers such as PSA [14]. Over expressed genes are now identified experimentally by using microarrays. Some of these genes have been proposed as candidate cancer biomarkers [15-17]. Despite this reasonable hypothesis, very few cancer biomarkers have been discovered by using this approach [17-18].
MARKERS OF DISEASE IN PROSTATE CANCER
There are no reliable biomarkers for disease progression in aggressive prostate cancer that has demonstrated utility in product development. Although prostate specific antigen (PSA) is used for a variety of purposes (e.g., determining when further diagnostic testing is indicated, assessing response to therapy), there is no consensus on how best to use PSA in cancer therapeutic trials. Uses of PSA that should be further investigated including identifying high-risk populations, providing an early marker of drug activity and dose range, and use of PSA as a marker of disease progression. Other markers may also prove more predictive of clinical outcomes in some patients (e.g., alpha methyl aryl CoA racemes expression as a predictor of disease progression in local disease). A gap analysis to rigorously identifying what is proven and unproven about PSA and other potential indicators would be an important first step to improving prostate cancer biomarkers.
Some established cancer biomarkers
|
Biomarker |
Cancer Type |
|
α-Fetoprotein (AFP) Carcinoembryonic antigen (CEA) PSA CA125 CA15.3 CA19.9 Chroriogonadotropin (hCG) |
Hepatoma; testicular cancer Colon; breast; lung; pancreatic Prostate Ovarian Breast Gastrointestinal Testicular cancer; trophoblastic tumors |
An unprecedented interest in biomarker development has arisen from the increasing use of genomic information and high-throughput technologies in the field of drug development.
There is an acute need for effective biomarkers in every phase of drug development, from discovery, to preclinical studies, through to clinical trials. The context in which these molecular biomarkers are used will depend upon the nature of the biological problem being addressed.
However, the process of biomarker identification and validation has historically been an arduous one. If high-throughput technologies are going to succeed in delivering safer, more efficacious drugs, they must also deliver superior biomarkers ensuring that success[19].
CONCLUSIONS
In the overall process of new drug development, it is clear that the target validation and preclinical assessment phases hold the strongest promise for clinical impact of genomics including the identification and application of new biomarkers.
This review has focused on the potential to identify novel biomarkers derived from genomic information as well as emerging high-throughput technologies. Biomarkers can be effectively used at multiple stages of the drug development process and need not necessary overlap in function.
A large concerted effort is required to advance the field of biomarker discovery. Most current biomarkers do not satisfy the required characteristics for use among the spectrum of diseases. Validation of new biomarkers is necessary.
REFERENCES
1. BIOMARKERS DEFINITIONS WORKING GROUP: Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. (2001) 69:89-95.
2. Thompson ML, Zucchini W. On the statistical analysis of ROC curves. Stat Med. 1989; 8: 1277-1290.
3. Thomson R. Nature Reviews Drug Discovery. 2007 FDA drug approvals: a year of flux. 2008; 7: 107.
4. U.S. Food and Drug Administration. Innovation or stagnation: challenge and opportunity on the critical path to new medical products. Rockville, MD: U.S. Food and Drug Administration, U.S. Department of Health and Human Services; March 2004. www.fda.gov/oc/initiatives/criticalpath/whitepaper.html #execsummary.
5. Lesko LJ and Atkinson Jr AJ. Use of Biomarkers and Surrogate Endpoints in Drug Development and Regulatory Decision Making: Criteria, Validation, Strategies, Annu.Rev. Pharmacol. Toxicol. 2001; 41: 347-366.
6. FRANK R, HARGREAVES R: Clinical biomarkers in drug discovery and development. Nat. Rev. Drug Discov. (2003) 2(7):566-580.
7. MORRISH PK, RAKSHI JS, BAILEY DL, SAWLE GV, BROOKS DJ: Measuring the rate of progression and estimating the preclinical period of Parkinson’s Disease with [18F] dopa PET. J. Neurol. Neurosurg. Psychiatry (1998) 64(3):314-319.
8. DU AT, SCHUFF N, ZHU XP et al.: Atrophy rates of entorhinal cortex in AD and normal aging. Neurology (2003) 60(3):481-486.
9. Menon, U., and Jacobs, I. (2002) Screening for ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 16, 469–482.
10. Finne, P., Finne, R., and Stenman, U. H. (2001) Neural network analysis of clinicopathological factors in urological disease: A critical evaluation of available techniques. Brit. J. U. Intl. 88, 825–83.
11. Stephan, C., Vogel, B., Cammann, H., Lein, M., Klevecka, V., Sinha, P., Kristiansen, G., Schnorr, D., Jung, K., and Leoning, S. A. (2003) An artificial neural network as a tool in risk evaluation of prostate cancer. Indication for biopsy with the PSA range of 2–20 microg/l. Urologe A. 42, 1221–1229.
12. Welsh, J. B., Sapinoso, L. M., Kern, S. G., Brown, D. A., Liu, T., Bauskin, A. R., Ward, R. N., Hawkins, N. J., Quinn, D. I., Russell, P. J., Sutherland, R. L., Breit, S. N., Moskaluk, CA., Frierson, H. F., Jr., and Hampton, G. M. (2003) Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc. Natl. Acad. Sci. U. S. A. 100, 3410–3415.
13. Yousef, G. M., Polymeris, M. E., Yacoub, G. M., Scorilas, A., Soosaipillai, A., Popalis, C., Fracchioli, S., Katsaros, D., and Diamandis, E. P. (2003) Parallel overexpression of seven kallikrein genes in ovarian cancer. Cancer Res. 63, 2223–2227.
14. Makglara, A., Scorilas, A., Stephan, C., Kristiansen, G. O., Hauptmann, S.,Jung, K., and Diamandis, E. P. (2000) Decreased concentrations of prostate-specific antigen and human glandular kallikrein 2 in malignant versus nonmalignant prostatic tissue. Urology 56, 527–532.
15. Welsh, J. B., Sapinoso, L. M., Si, A. I., Kern, S. G., Wang-Rodriguez, J., Moskaluk, C. A., Frierson, H. F, Jr., and Hampton, G. M. (2001) Analysis of gene expression identifies candidate markers and pharmacological targets in prostate cancer. Cancer Res. 61, 5974–5978.
16. Welsh, J. B., Zarrinkar, P. P., Sapinoso, L. M., Kern, S. G., Behling, C. A., Monk, B. J., Lockhart, D. J., Burger, R. A., and Hampton, G. M. (2001) Analysis of gene expression profiles in normal and neoplastic ovarian tissue samples identifies candidate molecular markers of epithelial ovarian cancer. Proc. Natl. Acad. Sci. U. S. A. 98, 1176–1181.
17. Hellstrom, I., Raycraft, J., Hayden-Ledbetter, M., Ledbetter, J. A., Schummer, M., McIntosh, M., Drescher, C., Urban, N., and Hellstrom, K. E. (2003) The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 63, 3695–3700.
18. Kim, J. H., Skates, S. J., Uede, T., Wong, K. k. K. K., Schorge, J. O., Feltmate, C. M., Berkowitz, R. S., Cramer, D. W., and Mok, S. C. (2002) Osteopontin as a potential diagnostic biomarker for ovarian cancer. J. Am. Med. Assoc. 287, 1671–1679.
19. Wendy J Bailey & Roger Ulrich, Merck & Co., Inc., 770 Sumneytown Pike, West Point, PA 19486 USA.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









