{ DOWNLOAD AS PDF }
 ABOUT AUHTORS
ABOUT AUHTORS
Jaha Sultana Mohammed
Pelcat Formulation PVT ltd
sohnivya786@gmail.com
ABSTRACT:
Ion exchange chromatography is probably the most powerful and classic type of liquid chromatography. The popularity of ion exchange chromatography has been increased in recent years because this technique allows analysis of wide range of molecules in pharmaceutical, biotechnology, environmental, agricultural and other industries for water purification to separation of various antibiotics from fermentation broths which will enhance the yields and reduce the production time for industrial process. The main objective of this particular study is to develop some understanding for the process of ion exchange and helps to determine whether or not ion exchange will be useful for a particular application. This topic includes background, theory, instrumentation, application which covers both the production of the ion exchange substance, a resin and its operation depending on the condition of matrix during use.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2464
|
PharmaTutor (ISSN: 2347 - 7881) Volume 5, Issue 2 Received On: 22/09/2016; Accepted On: 28/09/2016; Published On: 01/02/2017 How to cite this article: Mohammed JS; A brief review on Ion Exchange Chromatography; PharmaTutor; 2017; 5(2); 30-38 |
INTRODUCTION:
Chromatography literally means “colour writing”. Ion exchange chromatography involves the separation of ionizable molecules based on their total charge. This technique enables the separation of similar types of molecules that would be difficult to separate by other techniques because the charge carried by the molecule of interest can be readily manipulated by changing buffer pH. Columns used for ion exchange are characterized by the presence of the charged group covalently attached to the stationary phase. Anion exchanger contains bounded positive groups, where as cation exchanger contains bounded negative groups.
BACKGROUND:
The phenomenon of ion exchange was first reported by two British Agricultural Chemists and date back to 1850. After the work of Gans in 1913 natural and synthetic (soils, clays and Zeolites) inorganic cation exchangers were used for softening of hard water. Modern ion exchange resins were first used by Adams and Holms in 1935 in which large variety of synthetic resins have been developed [1]. This technique has been developing since 19th century which was firstly used for purification of drinking water.
DEFINITION:
Ion exchange chromatography can be defined as a reversible process in which ions of same sign are exchanged between solid and liquid, a highly insoluble body in contact with it. The soil is known as an ion exchanger and no substantial organic polymer has been used as ion exchanger [1, 2]. It is also known as cation-anion exchange chromatography.
Ion exchange chromatography, which is also known as adsorption chromatography, is a useful and popular method due to it’s:
1. High capacity,
2. High resolving power,
3. Mild separation conditions,
4.Versatility and wide speared applicability,
5.Tendency to concentrate the sample relatively low cost [3].
PROPERTIES OF ION EXCHANGE CHROMATOGRAPHY:
The most common properties of all ion exchangers which have been used in analysis are:
* It should be almost insoluble in water and organic solvents, like benzene, carbon tetrachloride, ether etc.
* It should be complex in nature and in fact they are polymeric.
* It should contain active or counter ions that will leads to exchange reversibility with other ions in a surrounding solution without any substantial change in the material [9-12].
ADVANTAGES:
* Detectability: Useful for the detection of many inorganic salts and also for the detection of organic ions with poor UV absorptivity likes alkyl amines or sulfonates.
* Preparative separations: Usually preferred because of the availability of volatile buffers. Volatile buffer makes the removal of mobile phase easier.
* Useful to resolve very complex samples i.e., in the case of multi-step separation.
* Useful for separation of mixture of biological origin, in organic salts and some organometallics.
* Provides long life to resin [1, 2].
* Cheap maintenance
* Environmental friendly because it deals only with substance occurring in water.
THEORY:
The principle of separation is by reversible exchange of ions between the ions present in the solution and those present in the ion exchange resin. Ion exchange separations are mainly carried out in columns packed with an ion exchanger. There are two types of ion exchanger, namely
1. Cationic exchangers
2. Anionic exchangers
Cationic exchangers:
It possesses negatively charged groups and these will attract positively charged groups. These exchangers are also called acidic ion exchange materials since their negative charges result from the proteolysis of acidic groups
Anionic exchangers:
It has positively charged groups, which will attract negatively charged molecules. This exchanger is termed as basic ion exchange materials since their positive charges generally result from the association of protons with basic groups. Based upon the affinity of ions towards the matrix the ions like cation and anion are separated. The ions that have less affinity towards matrices will elute first and the ions that have more affinity towards matrices it will elute later [3-12].

The actual ion exchange mechanism is thought to be composed of five distinct steps:
1. Diffusion of the ion to the exchanger surface. This occurs very quickly in homogeneous solutions.
2. Diffusion of the ion through the matrix to the exchanger site. This is dependent upon the degree of cross linkage of the exchanger and the concentration of the solution.
3. Exchange of ions at the exchange site occurs. This occur instantaneously in an equilibrium process:
Resin - SO3H + Na+----------------->Resins - SO3Na + H+
Resin - N(CH3)3OH + Cl- ----------->Resin - N(CH3)3Cl + OH-
4. Diffusion Of the exchanged ion through the exchanger to the surface
5. Selective desorption by the eluent and diffusion of the molecule into the external solution takes places [12-14].
ION EXCHANGERS:
Ion-exchange processes are used to separate and purify metals, including separating uranium from plutonium and other actinides, including thorium and lanthanum, neodymium, ytterbium, samarium, lutetium, from each other and the other lanthanides.
There are three classes of ion exchangers, these includes
1. Resins
2. Gels
3. Inorganic exchangers
RESINS:
Resins are amorphous particles of organic materials which are composed of polystyrene and divinely benzene. Polystyrene contains sites for exchangeable functional groups. Divinely benzene acts as a cross linking agents and offers adequate strength i.e., mechanical stability. Ion exchange resins are used for separation of small molecules.
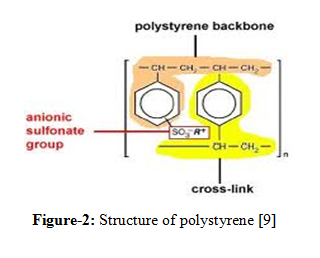
Classification of resins:
I. According to their chemical nature, they can be classified as
1. Strong cation exchange resin: These types of resins are useful for the chromatographic separation of amino acids, rare earths and other substances that contains sulphonic acid groups as the ionisable groups [1-12].
2. Weak cation exchange resin: These resins are based on polymers of methacrylic acid and possess carboxyl groups [1-12].
3.Strong anion exchange resin: These resins with positively charged quaternary ammonium groups attached to cross linked polystyrene frame work belong to this class. Trimethyl ammonium groups are used for this resin [1-12].
4. Weak anion exchange resin: The tertiary amine resins and polyamine resins having a mixture of primary, secondary and tertiary amine groups on the polystyrene network are well known [1-12].
II. According to the source, they are classified as
1. Natural source: These are of two types
a. Cation: E.g: Zeolytes, clay etc
b. Anion: E.g: Dolomite
2. Synthetic source: These are of two types
a. Inorganic and
b. Organic resins.
Of the above types, organic resins are the most widely used [14].
General requirements of resins:
It should be chemically stable.
It should be insoluble in most of the common solvents.
It should have high degree of exchange of ions.
If it swells then it should be denser than water molecule.
It must contain sufficient number of ion exchange groups.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
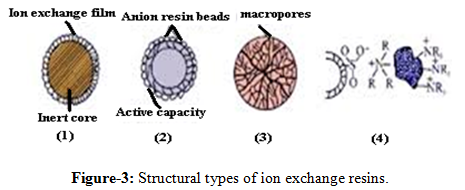
III. Structural types of ion exchange resins
1. Pellicular type with ion exchange film:
The particle size is ranging from 30 - 40 mm with 1 - 2 mm film thickness. These have very low ion exchange capacity to separate the ions. Their ion exchange efficiency is 0.01 - 0.1 meq/g of ion exchange capacity [15].
2. Porous resin coated with exchanger beads:
The particle size ranges from 5 – 10m. They are totally porous in nature and highly efficient. Their exchange capacities are from 0.5 - 2 meq/g of ion exchange resin [15].
3. Macro reticular resin bead:
A reticular network of the resin is seen superficially on the resin beads. They are not highly efficient and have very low ion exchange capacities [15].
4. Surface sulfonated and thermostatically bonded with anion:
It is less efficient and also has less exchangeable capacity [15].
GELS: Ion exchange gels are used for the separation of large molecules like proteins, nucleic acids. Cellouse and dextran ion exchangers which are polymers of sugar glucose possess large pore sizes and lower charge densities. Because they are much softer than polystyrene resins, dextron and its relatives are called as gels.
INORGANIC EXCHANGERS: The combinations of hydrous oxides of highly charged ions, with one oxide more acidic than the other have been found to have ion exchanging properties. The amorphous precipitates have higher exchange capacities than the crystalline compounds because of the greater surface area of the former type of compounds. Inorganic exchangers are employed when Separations involving harsh chemical conditions such as high temperature, high radiation level, strong basic solution or powerful oxidizing agent. E.g.: Titanium Arsenite has been used to absorb alkaloids. Hydrous antimony peroxide has been used to study exchange equilibrium of Ka and Rb ions with hydrogen and other ions [12].
INSTRUMENTATION:
- General components of an ion-exchange chromatography are presented as below (Figure - 4) [12].
- A high pressure pump with pressure and flow indicator, to deliver the eluent.
- An injector for introducing the sample into the eluent stream and onto the column
- A column, to separate the sample mixture into the individual components
- An oven, optional
-A detector, to measure the analyte peaks as eluent from the column
- A data system for collecting and organizing the chromatograms and data [12].

Figure – 4: Schematic Diagram of Ion exchange chromatography
Column:
Column used in the laboratories are made up of glass but those used in industries are made up of either high quality stainless steel or polymer, which are resistant to strong acids and alkalis [5]. The geometry of column depends completely on the separation factor. The separation is improved by increasing the length of the column but the length cannot be increased beyond a critical length. Uneven flow of liquid is possible in case of column too wide or too narrow in size [16]. A Dimension of column is 20:1 to 100:1 for the higher efficiency can be used [15].
Packing of the column:
In this wet packing method is used. The resins is mixed with the mobile phases and packed in the column uniformly. The sample to be separated is dissolved in the mobile phases and introduced all at once into the column [15].
Application of the sample:
After packing the column, the solution to be analyzed is added to the top of the column and allowed to pass through the bed of ion exchanger. For this purpose the syringe or pipette is utilized [17].
Mobile phase:
The organic solvents are less useful so they are not used these days. Only different strength of acids, alkalis and buffer are used as eluting solvent [11-17]. E.g.: 0.1 N HCl, 1N NaOH, phosphate buffers, acetate buffers, borate buffers, phthalate buffers, etc.
Developments of the chromatogram and elution:
After introduction of the sample, development of the chromatogram is done by using different mobile phases. The aqueous salt solution is adjusted to a constant ionic strength. The choice of the mobile phase depends on the selectivity of the resin for the solute ions. Two types of elution techniques are used:
a. Isocratic elution
b. Gradient elution
In Isocratic elution technique, the same solvent composition is used i.e., same strength of acids or alkalis or buffers are used.
In gradients elution techniques, initially less acidic or basic mobile phase is used. Then, acidity or basicity is increased at regular intervals. The different fraction of the elution is collected volume wise or time wise and analyzed [15].
Analysis of the elute or Detection:
Different fractions are collected with respect to the volume or time is analyzed for their contents. Several methods of analysis can be used which depends upon the nature and quantity of the ionic species are:
* Conductometric method
* Ampherometric methods
* Flame photometric method
* Polarography
* UV. Spectroscopy
* Radiochemical methods using Geiger Muller counter, ionization chamber method [16-18].
Conductivity detector is the most common and useful detector in ion exchange chromatography. Conductivity detection gives excellent sensitivity when the conductance of the eluted solute ion is measured in an eluent of low background conductance. Therefore when conductivity detection is used dilute eluents should be preferred and in order for such eluents, to act as effective competing ions, the ion exchange capacity of the column should be low [1]. The results obtained are stored in computer.
Regeneration of ion exchange resin:
The ion exchange resin after separation may not be useful for next separation as exchange functional groups are lost. But due to the cost of ion exchange resins, they cannot be disposed off. Hence reactivation, regeneration of the resins is most important. Regeneration makes the used ion exchange resins to be as efficient as virgin resins. Regeneration refers to the replacement of the exchangeable cations or anions present in the resin. Hence the charging of the column with strong acid like hydrochloric acid is used for regeneration of the cation exchange resin, using strong alkali like sodium hydroxide or potassium hydroxide also used for regeneration of anion exchange resin [8-14].
ION EXCHANGE TECHNIQUES
Two techniques are generally used to bring the solution in contact with ion exchange resins. They are:
1. Batch method
2. Column method
1. Batch method:
This method involves single step equilibrium. The resin and the solution are mixed in a vessel until equilibrium is attained. The solution which is obtained filtered. In single step equilibrium of this type, only a single portion of the exchange capacity of the resin is utilized. This method is used for softening of water and the production of deionised or demineralised water [14-17].
2. Column method:
Ion exchange chromatography is technique in which separation of the components of a mixture is based upon the different selective coefficients for the resin which leads to different migration rates on an ion exchange column. Chromatography process is classified according to physically state of mobile and stationery phases, whether they are gases, liquid and gases [12]. This are
1) Frontal analysis,
2) Elution analysis and
3) Displacement development [16]
The apparatus used in the column method consist of a glass column fitted with a glass wool plug or a sintered glass disc at the lower end. An ordinary burette can also be used. The resin used should have small particle size and the diameter of the resin should be less than one tenth of the column. The slurry is slowly poured into the column containing some water. The resin is allowed to settle and then excess water is drained off [1].
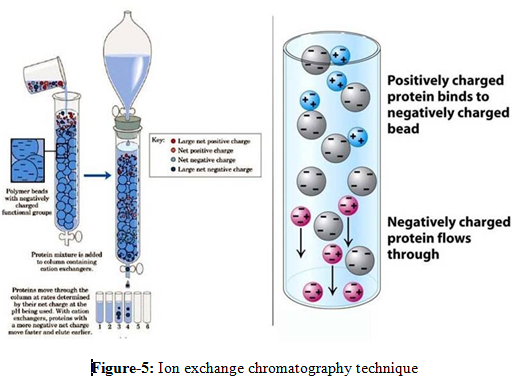
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
APPLICATIONS: Ion exchange chromatography can be applied for the separation and purification of many charged or ionisable molecules such as proteins, peptides, enzymes, nucleotides, DNA, antibiotics, vitamins and etc. from natural sources or synthetic origin [14]. Some of its applications are as follows:
1. Separation of similar ions: The ion exchange chromatography is used for separation of similar ions as different ions undergo exchange reactions to different extent [13-16]. E.g.: A mixture of H+, Na+, and K+ can be separated by using cation exchange resin. Similarly Cl-, Br-, I- can be separated by passing through basic anion exchanger.
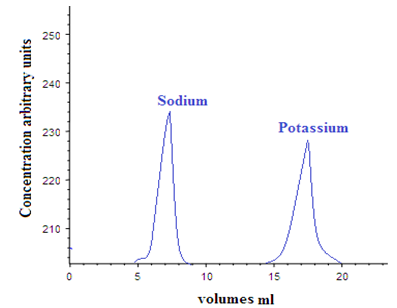
Graph-1: Separation of potassium from sodium on Dowex 50 x 12 by elution with 0.6 M HCl
2. Softening of hard water: Hardness of water is due to the presences of Ca2+,Mg2+ and other divalent ions may be removed by passing the hard water through the cation exchanger charged with Na+ions. Then the following exchange reaction takes place:
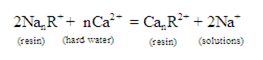
TheCa+, Mg+ ions from water are retained on the column while Na + ions pass into the solution. These Na+ ions are harmless for washing purpose. After using the ion exchange for a time, it becomes in active. Percolating through it a concentrated solution of NaCl when the following reverse reaction takes place can revive its activity [12-16].
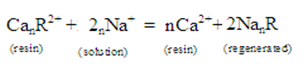
3. Complete demineralization of water: This requires complete removal of ions i.e., both cations and anions. For this, water is passed through an acidic cation exchanger then metallic cations are exchanged with H+ ions. The water obtained is then passed through a basic anion exchanger then the anions present in the water are exchanged by OH- of the exchanger. The H+and OH- ions which pass into solution combine to form unionized water [5, 12, and 14]. E.g.: Cation exchanger – Sulphonic acid resin is commonly used Anion exchanger – Strong basic resin is used
4.Purification of organic compounds: Many natural products extracted in water have been found to contain ions originally present in water. Those ions can be removed by using ion exchange process [17].
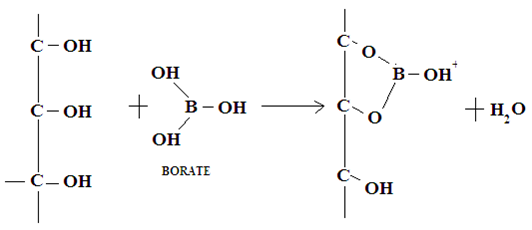
5. Separation of sugars: This method is developed by Khym and Zill in 1951 [17]. Sugars are first converted into borate complex and the separation of borate complexes have been achieved quantitatively on columns by using ion exchange chromatography. In this, disaccharide can be separated from monosaccharide’s and the individual compounds of hexose, pentose from the mixture can be resolved [6, 12].
6. Separation of amino acids: Ion exchange methods can be used to separate the complex mixture of 18 amino acids obtained by the acid hydrolysis of proteins. The mixture of amino acids is first introduced on a very short column at pH 2 and eluted with 0.35 N sodium citrate buffers at pH 5.25. Acidic and neutral amino acids at first leave the column unseparated and after that other amino acids are separated [17]. Similarly, a mixture of vitamins like vitamin B1, B2, B6, Niacin, Folic acid, B12 etc. can be separated using ion exchange technique [16].

7. Purification and recovery of pharmaceuticals:
The process is used for purification and recovery of antibiotics, vitamins, alkaloids, hormones and other chemicals of pharmaceutical importance during their manufacturing process [17].
8. Medicinal importance:
Anionic resins are introduced in the treatment of ulcer while cation exchangers have been used to remove Na+ from body during the treatment of hypertension and edema. The resins are also used as a diagnostic aid in gastric acidity tests. The resins have been successfully used with other medicinal agents to achieve delayed action dosages [17].
9. Biochemical separations:
Used for biochemical separations like some drugs or metabolites from blood, urine or other biological fluids [9, 13].
10. Ion exchange column in HPLC:
For separation of compounds of mixed nature like acidic and basic substances, ion exchange is used in HPLC [14].
11. Concentration of ionic solutions:
A cation or anion from a bulk of solution can be adsorbed onto ion exchange resins, after adsorption, it can be eluted by using small volume of eluent [9].
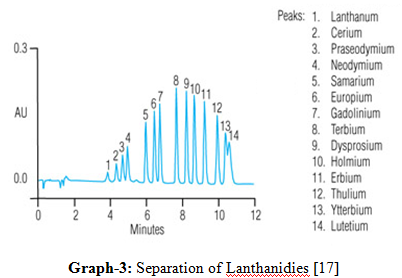
12. Separation of lanthanides:
Solution having mixture of lanthanides is passed through a column packed with particles of a suitable ion exchange resin. Cations present in solution undergo exchange with hydrogen with hydrogen ions [12, 16, and 17].
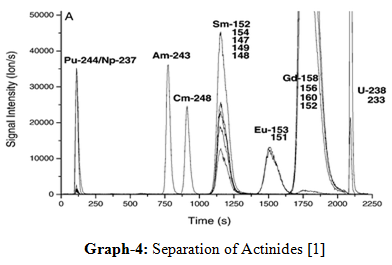
13.Separation of actinides:
The IEC technique has played a unique role in the discovery on the Trans plutonium elements in the actinide series. The power of the method can be judged from the order of elution of lanthanides (4f) and actinide (5f) ions in the +3 oxidation state from a cation exchange resin column with an aqueous solution of ammonium hydroxyl isobutyrate. In the actinide series also the elution occur in the reverse order of the atomic number due to actinide contraction and this proved that IEC is only way for identifying these elements [1, 8, 11, 16].
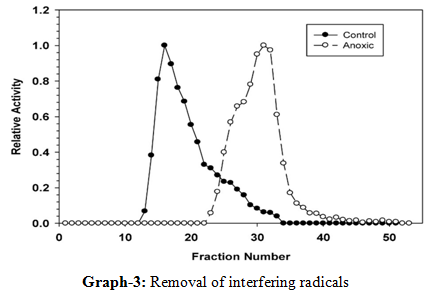
14. Removal of interfering radicals: The estimation of Ca+ or Ba+ ions is carried out by the oxalate or sulphate method in which phosphate ion is found to interfere. Therefore, its removal becomes necessary which is achieved by passing a solution of Ca+ or Ba+ ions through suitable ion exchanger in the column. The process has to be repeated so that the phosphate ions are completely removed. Now, the calcium and Ba+ ions held by resin will be removed by using suitable eluent. Finally, these ions are estimated by the usual methods [12, 17, and 18].
15. Other applications:
* For the measurement of various active ingredients in medicinal formulations.
* For the measurement of drugs and their metabolites in serum and urine, for residue analysis in food raw materials.
* For the measurement of additives such as vitamins and preservatives in food and beverages
LIMITATIONS:
1)Column efficiency is less.
2)It is difficult to achieve control over selectivity and resolution.
3)Stability and reproducibility of the columns become questionable after repeated use.
4)Nature of exchanging ions is not known.
5)There are substance, such as organic matter Fe3+occurring in some water which can foul the resin
CONCLUSION:
Several different types of liquid chromatography techniques are utilized for isolation of bioactive molecules from different sources. Follow-up of the nonsolvent extractable natural products can be realized by this technique. Consequently ion exchange chromatography, which has been used in the separation of ionic molecules for more than half a century is still used as an useful and popular method for isolation of natural products in modern drug discovery and it continue to expand with development of new technologies.
REFERENCES:
1. B.K. Sharma; Instrumental Methods of Chemical Analysis; page. No: 123-160.
2. Willard Merritt Dean Settle; Instrumental Methods of Analysis; 7th edition; page. No: 633-639.
3. Kenneth A. Connors; A Text Book of Pharmaceutical Analysis; 3rd edition; page. No: 398 - 408.
4. Amersham pharmacia biotech; Ion Exchange Chromatography: Principles and Methods.
5. P. Parimoo; Pharmaceutical Analysis; page. No: 284 - 286.
6. Harvard apparatus; Guide to Ion Exchange Chromatography.
7. Tosoh Bioscience; Ion Exchange Chromatography.
8. Yasser M. Moustafa and Rania E. Morsi; Ion Exchange Chromatography - An Overview.
9. Dr. S. Ravi Sankar; Text book of pharmaceutical analysis; 4th edition; page. No: 16-2 – 16-10.
10. ROBERT D. Braun; Introduction to Instrumental Analysis; page. No: 850-859.
11. R. A. Day, A. L. Underwood; Quantitative Analysis; 6th edition; page. No: 535-543.
12. J. Mendham, R.C Denney, J.D Barnes, M. Thomas, B. Siva Sankar; Text Book of Quantitative Chemical Analysis, 6th edition; page. No: 213-222.
13. G. Vidya Sagar; A Text Book of Pharmaceutical Analysis; volume-II; page. No: 13 – 18.
14. Anees A. Siddiqui; Pharmaceutical Analysis; volume-I;page. No: 216 – 236.
15. Gurdeep R. Chatwal, Sham K. Anand; Instrumental Methods of Chemical Analysis; page. No: 2.662-2.672.
16. Dr. A. V. Kasture, Dr. K. R. Mahadik, Dr. S. G. Wadodkar, Dr. H. N. More; Pharmaceutical Analysis; volume-II; page. No: 39 – 47.
17. G. DevalaRao; A Text Book of Pharmaceutical Analysis; volume-II; page. No: 54 – 57.
18. Mianbin Wu, Xuewan Wang, Zhengyu Zhang and Rutao Wang; Isolation and Purification of Bioactive Proteins from Bovine Colostrum.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









