About Author: Ambrish Singh
B.Pharma, M.Tech (Biotech)
Anna University,
Chennai
Abstract
Products which degrade upon disposal by the action of living organisms are called 'biodegradable products”. They can be broken down into their constituent natural elements and be absorbed by the environment. There has been a lot of progress in the production of biodegradable products from biopolymers such as starch, cellulose, lactic acid, glycolic acid etc. Biopolymers have a vast range of applications in the production of eco- friendly products such as bioplastics, biopots, biodegradable medical devices, biodegradable implants and drug delivery systems. Using biodegradable products is very important for making the environment “Green”. Advanced techniques in biotechnology have been used to produce genetically modified organisms. These genetically modified organisms are used in the production of biopolymers. Biopolymers help to produce biodegradable products in large quantities at a very low cost. Therefore focusing research in these areas will help to completely avoid the usage of non biodegradable products in future.
Reference ID: PHARMATUTOR-ART-1104
Introduction
The ASTM (The American Society of Testing and Materials) defines 'biodegradable' as “capable of undergoing decomposition into carbon dioxide, methane, water, inorganic compounds, or biomass in which the predominant mechanism is the enzymatic action of microorganisms, that can be measured by standardized tests, in a specified period of time, reflecting available disposal condition”. Biodegradable products are mainly produced from biopolymers. Biopolymers are used in packaging materials (trash bags, wrappings, loose-fill foam, food containers, film wrapping, laminated paper), disposable nonwovens (engineered fabrics) and hygiene products (diaper back sheets, cotton swabs), consumer goods (fast-food tableware, containers, egg cartons, razor handles, toys), and agricultural tools (mulch films, planters). Biodegradable products are very useful in medical applications. Polymers prepared from glycolic acid and lactic acids have found a multitude of uses in the medical industry, beginning with biodegradable sutures first approved in the 1960s. Since then, medical devices have been produced using lactic and glycolic acidand on other materials, including poly (dioxanone), poly (trimethylene carbonate) copolymers, and poly (?-caprolactone) homopolymers and copolymers. In addition to this, extensive research is being carried out on polyanhydrides, polyorthoesters, polyphosphazenes, and other biodegradable polymers. Synthetic biodegradable polymers can be used in tissue engineering and controlled drug delivery. Biopolymers have many properties and applications. A polymer is a long molecule containing many repeated units in covalent bonds. A biopolymer is a “polymer of natural origin” and can include such diverse materials as wood, cellulose, and chitin. They are generated from renewable sources and are easily biodegradable because of the oxygen and nitrogen atoms found in their structural backbone. Various experts have classified biopolymers differently; however, the United States Congress Office of Technology Assessment separates them into nucleic acids, proteins, polysaccharides, polyhydroxyalkanoates, and polyphenols. The divisions are based on the chemical organization of basic monomers within the biopolymers. Proteins, polysaccharides, polyhydroxyalkanoates, and polyphenols have many uses in industry, medicine, construction, etc.
Need for a Biodegradable Products
Conventional polymers such as polyethylene and polypropylene persist for many years. These polymers are not appropriate for applications in which plastics are used for short time periods and then disposed. Further, plastics are often soiled by food and other biological substances, making physical recycling of these materials impractical and generally undesirable. As per reports of various environment protection agencies, plastics alone account for more than 25 % (by volume) of municipal waste generated. Low density and slow decomposition makes plastic a visible pollutant of public concern (Fig 1). Some of the techniques adopted for integrated waste management, which include recycling, source reduction of packaging materials, composting of degradable wastes, incineration etc., may help reduce waste disposal problem; but this will not solve the problems of importation of petroleum products non-degradability of plastics. As per statistics, about 80 % of postconsumer plastic waste is sent to landfill which degrade land masses and cause water pollution, 8 % is incinerated causing unwanted emission and only 7 % is recycled. In some countries the situation is very bad leaving very few sites that can be used for landfill. The main bulk of domestic waste is made up of plastics. This has generated a great deal of interest in recycling plastics and in producing plastic materials that can be safelyand easily disposed off in the environment.
Non-degradable products can be used to make bio-degradable products suitable for various applications. This would help reduce the adverse effects of non degradable products. When biodegradable products are disposed in bioactive environments, they are degraded by the enzymatic action of microorganisms such as bacteria, fungi, and algae. Their polymer chains may also be broken down by nonenzymatic processes such as chemical hydrolysis. When plants process atmospheric CO, they 2 lead to the production of biopolymers. Biodegradation converts them to CO, CH, water, biomass, humic ma and other natural substances. 2 4 Biopolymers are thus naturally recycled by biological processes. Fig 2 shows the cyclic process by which agricultural and fermentative routes can yield biodegradable biopolymers (Gross RA and BhanuKalra, 2002).
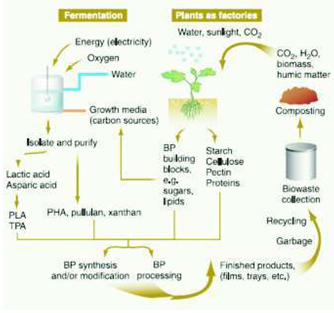
Fig 2: Cyclic Process by which agricultural products and fermentative routes can yield biodegradable biopolymer (Courtesy: Gross A. R and Bhanu Kalra, 2002)(PLA- Poly lactic Acid; TPA- Thermal polyaspartates, PHA- Poly hydroxyl alkanoates, BP-Biopolymer)
Production of Biodegradable Products from Biopolymers
Biodegradable Plastics
Biodegradable plastics can be produced from starch, cellulose, poly hydroxyl alkanoates, poly lactic acid, and poly (?-caprolactone). Starch is an inexpensive, annually renewable material derived from corn and other crops. The biodegradation of starch products help in recycling atmospheric CO trapped by starch-producing plants. Starch-based 2 biopolymers can be produced by blending or mixing them with synthetic polymers. The morphology and properties of starch - based biopolymers can be regulated easily and efficiently by varying the synthetic blend component and its miscibility with starch. Blends containing thermoplastic starch (destructurized starch that is noncrystalline, produced by the application of heat and work) may be blended or grafted with biodegradable polyesters (such as polycaprolactone) to increase flexibility and resistance to moisture. These materials are mainly formed into films and sheets. Blends with more than 85 % starch are used for foaming and injection molding. The foams can be used as loose-fill in place of polystyrene; Loose-fill materials from starch are generally water sensitive. Rigid and dimensionally stable injection-molded articles are produced when thermoplastic starch is mixed with cellulose derivatives.
Chemically modified cellulose is used in many common applications. For example, cellulose acetate is used in many common applications, including toothbrush handles and adhesive tape backing (Gross and Bhanu Karla, 2002).
Poly Lactic Acid (PLA) is produced from lactic acid by solvent-free continuous process and distillation method. Upon disposal, PLA degrades primarily by hydrolysis, not microbial attack. Hence, even at high humidity, PLA with high molecular weight does not get easily contaminated by fungi, mold, or other microbes. Biopolymers are therefore used in application in which they are in direct contact with foods for extended time periods. PLA can be converted into compost in municipal compost facilities. PLA can be thermally processed using standard machinery. PLA is currently used in packaging (film, thermoformed containers, and shortshelf life bottles). Fabrics produced from PLA are silky, durable, and moisture resistant, keeping the wearer dry and comfortable. Fig 3 shows the recycling of biodegradable products produced from PLA.
Poly (?-caprolactone) (PCL) is thermoplastic biodegradable polyester synthesized by chemical conversion of crude oil, followed by ringopening polymerization. PCL is highly resistant to water, oil, solvent, and chlorine, it has a low melting point, and viscosity, and can be easily processed thermally. To reduce manufacturing costs, PCL may be blended with starch - for example, to make trash bags. Scrub-suits, incontinence products, and bandage holders have been produced by blending PCL with fiber forming polymers (such as cellulose), hydro entangled nonwovens (in which bonding of a fiber web into a sheet is accomplished by entangling the fibers by water jets). The rate of hydrolysis and biodegradation of PCL depends on its molecular weight and degree of crystallinity. However, PCL can be biodegraded completely by many microbes. This and related polyesters are water resistant and may be melt-extruded into sheets, bottles, and various shaped articles. Hence, these plastics are primary targets for use as biopolymers. Many biodegradable polyesters are now in the market or at an advanced stage of development.
Biodegradable Pots
Biodegradable pots are produced by injection moldings from biodegradable plastics. Gardeners and farmers can place potted plants directly into the ground, and forget them. The pots will break down to carbon dioxide and water, eliminating double handling and recycling of conventional plastic containers.
Water-Soluble Biodegradable Polymers
Water-soluble polymers are used as detergent builders, scale inhibitors, flocculants, thickeners, emulsifiers, and paper-sizing agents. They are used in cleaning products, foods, toothpaste, shampoo, conditioners, skin lotions, and textiles. Water-soluble biopolymers may be synthesized by modifying starch and cellulose. For example, carboxymethyl cellulose having different degrees of carboxymethyl substitution is a family of marketed water-soluble polymers. Hydroxyethyl cellulose is used as thickeners in drilling fluids and as fluid-loss agents in cementing. polysaccharide-derived polymers, the rate or extent of product biodegradability decreases when higher levels of modification of cellulose (more than one substituent per sugar ring) are required to achieve desired performance characteristics to produce such polysaccharide-derived polymers. Further, many of these polymers have not yet been studied by standardized testing methods to determine their biodegradability (Van et al., 1996).
Water - soluble polysaccharides are also produced by microbial fermentation. Xanthan is the most widely used microbial polysaccharide. Industrial uses of xanthan include oil recovery (viscosity control), paper manufacturing, agriculture (stimulation of plant growth), and cosmetics. Pullulan has shown a plethora of applications. For example, its good moisture retention and low oxygen permeability has led to its use as edible films for food packaging.
Poly (amino acids) with free carboxylic groups, such as poly (aspartic acid) and poly- (glutamic acid) can be used as water-soluble biopolymers. Polymers based on aspartic acid have attained the greatestcommercial success. Poly (aspartic acid) (thermal polyaspartate) may be produced in two different ways. They are functionally equivalent to poly (acrylic acid) and, when highly linear, are fully biodegradable. Thermal poly aspartates are being targeted globally mainly to produce performance chemicals, diapers, and in agriculture. In agriculture, thermal polyaspartates have been found to stimulate crop growth by enhancing root development. Poly (malic acid), the polyester equivalent of poly (aspartic acid), may be used to produce biodegradable detergents. However, these polymers are hydrolytically unstable. To date, poly (vinyl alcohol) is the only polymer with exclusively carbon atoms in the main chain that is regarded as biodegradable. It is currently used in textiles, paper and packaging industries as paper coatings, adhesives, and films (Wolk et al., 1994).
Synthetic Biodegradable Polymers as Medical Devices
Biodegradable polymers can be either natural or synthetic. In general, synthetic polymers offer greater advantages over natural materials. Synthetic polymers can be used to produce materials with a wider range of properties and having more lot-to-lot uniformity. Synthetic polymers also represent a more reliable source of raw materials free from concerns of immunogenicity. Synthetic biodegradable polymers are currently being used or investigated for use in wound closure (sutures, staples); orthopedic fixation devices (pins, rods, screws, tacks, ligaments); dental applications (guided tissue regeneration); cardiovascular applications (stents, grafts); and intestinal applications (anastomosis rings). Most of the commercially available biodegradable devices are polyesters composed of homopolymers or copolymers of glycolide and lactide. There are also devices made from copolymers of trimethylene carbonate and ?-caprolactone, and a suture product made from polydioxanone.
Polyglycolide (PGA) was used to develop the first totally synthetic absorbable suture. Glycolide monomer is synthesized from the dimerization of glycolic acid. Materials with high molecular weight are derived by ring-opening polymerization. Sutures of PGA lose about 50 % of their strength after 2 weeks and 100 % at 4 weeks, and are completely absorbed in 4-6 months. Glycolide has been copolymerized with other monomers to reduce the stiffness of the resulting fibers.
Polylactide (PLA) materials exhibit high tensile strength and low elongation. Consequently, they have a high modulus that makes them suitable for load-bearing applications in orthopedic fixation and sutures. Poly (dl-lactide) is an amorphous polymer exhibiting a random distribution of both isomeric forms of lactic acid, and accordingly is unable to arrange itself into an organized crystalline structure. This material has lower tensile strength, higher elongation, and a much more rapid degradation time, making it more attractive as a drug delivery system. LPLA takes longer to degrade than DLPLA. LPLA requires more than two years to be completely absorbed.
Poly (dl-lactide) is used in two dental applications. It is employed as void filler following tooth extraction, porous polymer particles can be packed into the cavity to aid in quicker healing. As a guided-tissue-regeneration (GTR) membrane, films of biodegradable polymer can be positioned to exclude epithelial migration following periodontal surgery. The exclusion of epithelial cells allows the supporting, slower-growing tissue-including connective and ligament cells-to proliferate.
Poly (?-caprolactone) The ring-opening polymerization of p-dioxanone resulted in the first clinically tested monofilament synthetic suture. Poly (dioxanone) has no acute or toxic effects on implantation. The monofilament loses 50 % of its initial breaking strength after 3 weeks and is absorbed within 6 months (Middleton and Tipton, 1998). The application of biopolymers with its degradation time is shown in Table 1.
|
Polymer |
Degradation Time |
Application |
|
PGA |
6-12 months |
Sutures, Anastomosis ring, Pins and rods |
|
LPLA |
>24 months |
Sutures, Interference screws, Suture anchor, Anastomosis clip, Screw, Pins and rods, Tack |
|
DLPLA |
12-16 months |
Sutures, dental, plates, mesh, screws, guided tissue |
|
PCL |
>24 months |
Sutures |
|
PDO |
6 to 12 months |
Sutures |
|
PGA-TMC |
6 to 12 months |
Suture anchor |
Table 1. application of biopolymers in producing medical devices
Biodegradable Implants
For many years, biodegradable implants have been thought to offer advantages over metal analogs. In orthopaedic practice, metal implants can distort magnetic resonance imaging (MRI), and they release metal ions into the surrounding tissue. Another disadvantage is the need for a second surgical procedure for implant removal and complicated revision surgery resulting from the presence of the implant. The intent of biodegradable implants (Fig 4) is to provide secure initial fixation strength while allowing degradation and replacement by the host tissue. Here, there is no need for implant removal, revision surgery is not compromised, and radiological imaging is not distorted. In addition, functional loads can be assumed earlier by the healing bone while the material is degrading (Disegi and Wyss, 1989).
Approximately, 40 different biodegradable polymers are known (Claes, 1992). Of these, studies have been carried out on the following materials used in orthopaedic implants: Polyglycolide and copolymers such as polyglycolide-co-trimethylene carbonate, poly-(D,L-lactide-coglycolide) and poly-(L-lactide-co-glycolide), poly-(L-lactide), poly- (D,L-lactide), and their stereocopolymers with varying ratios of the L and D,L parts, polydioxanone. trimethylene carbonate, polyorthoester and poly-c-capralacton.
In principle, synthetic biodegradable polymers consisting of polyhydroxy acids undergo an unspecific hydrolysis water uptake. Degradation starts at the amorphous phase of the implant leading to fragmentation of the material to smaller parts, which are phagocytosed primarily by macrophages and polymorphonuclear leukocytes (Lam et al., 1993) Polymeric lactic acid oligomers degrade to monomers which enter the Krebs cycle and get dissimilated to carbon dioxide and water. Besides from the hydrolytic chain scission, glycolic acid monomers can be released by unspecific esterases and carboxypeptidases (Hollinger and Battistone, 1986).
Degradation kinetics of different raw materials differs substantially, which may be attributed to the hydrophilic or hydrophobic nature of the different polymers. Further, although the degradation kinetics of biodegradable implants depend primarily on polymer choice, a large variety of additional factors also appear to contribute to this processmolecular weight, sterilization, implant size, self-reinforcement, and processing techniques (Weiler et al., 2000).
Bio polymers for Drug Delivery
Biodegradable polymers being developed in Atlanta, USA, offer numerous advantages over current drug delivery systems. Research is being carried out on ways to deliver anti-inflammatory drugs directly into cells. Polyketals are hydrophobic polymers with biodegradable ketal linkages in their backbone. Polyketals form nanoparticles that can encapsulate either hydrophobic drugs or proteins. Polyketals have an advantage over current biodegradable polymers for drug delivery, such as polyesters. Polyesters release acidic byproducts as they degrade in the body, which can cause inflammation. Polyketals do not release inflammatory byproducts. Hence, polyketols score over polyesters.
Injectable, biodegradable scaffolds are important biomaterials for tissue engineering and drug delivery. Hydrogels derived from natural polysaccharides are ideal scaffolds as they resemble the extracellular matrices of tissues comprised of various glycosaminoglycans. A new class of biocompatible and biodegradable composite hydro gel is produced by mixing water-soluble chitosan and oxidized hyaluronic acid, without the addition of a chemical crosslinking agent. The gelation is attributed by N-succinyl-chitosan and aldehyde hyaluronic acid. The composite hydrogel supported cell survival and the cells retained chondrocytic morphology. These characteristics provide a potential opportunity to use the injectable, in situ forming biodegradable composite hydrogels in tissue engineering applications (Huaping Tan et al., 2008).
Biodegradable nanoparticles have been used frequently as drug delivery vehicles due to their bioavailability, better encapsulation, control release and less toxic properties. Biodegradable nanoparticles such as poly (D, L -lactide-co-glycolide), poly lactic acid, chitosan, gelatin, polycaprolactone and poly-alkyl-cyanoacrylates can be used to produce various nanoparticulate systems and to synthesise and in the encapsulation process. It can be also used to control release and in the improvement of therapeutic value of nanoencapsulated drugs (Avnesh Kumari et al., 2010).
Biodegradable nanoparticles formulated from poly (D, L -lactide-coglycolide)
have been extensively investigated for sustained and targeted/localized delivery of different agents including plasmid DNA, proteins and peptides and low molecular weight compounds. Research about the mechanism of intracellular uptake of nanoparticles, their trafficking and sorting into different intracellular compartments, and the mechanism of enhanced therapeutic efficacy of nanoparticleencapsulated agent at cellular level is more recent. Recent studies demonstrated rapid escape of PLGA nanoparticles from the endolysosomal compartment into cytosol following their uptake. Based on the above mechanism, biodegradable nanoparticles have various potential applications for delivery of therapeutic agents to the cells and tissue (Jayanth Panyam and Vinod Labhasetwar, 2003).
Conclusion
The development of biodegradable products has led to the greening of industrial production. Concerns about global climate change, pollution, dwindling stocks of petroleum, and other factors have led materials scientists to reconsider the virtues of bio-based materials. The production of biodegradable products from biopolymer represents a cleaner and safer way of making the environment clean. Breakthroughs in the genetic engineering of metabolic pathways have yielded microbes that efficiently convert inexpensive feed stocks (molasses, starch and waste lipids) to biopolymers. Cloning and expression of genes in plants has created new possibilities for using photosynthesis to directly synthesize polymers in plants. The continued development of costeffective biological routes can be developed using these powerful technologies. New biodegradable products may be developed using a wide range of biopolymers in future.
References
Avnesh Kumari, Sudesh Kumar Yadev and S.C. Yadav, 2010. Biodegradable polymeric nanoparticle based drug delivery systems. Bio.Mat., 75: 1-18.
Claes L.E. 1992. Mechanical characterization of biodegradable implants. Cli. Mat., 10: 41-46.
Disegi J.A. and H Wyss, 1989. Implant materials for fracture fixation: A clinical perspective. Ortho., 12: 75-79.
Gross A.R. and Bhanu Kalra, 2002. Biodegradable Polymers for the Environment. Sci., 297: 803 – 807.
Hollinger J.O. and G.C Battistone, 1986. Biodegradable bone repair materials. Syn. Cer.. Clin Orthop., 207: 290-305.
Huaping Tan, C.R. Chu , K.A. Payne and K.G. Marra, 2008. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomat., 30: 2499-2506.
Jayanth Panyam and Vinod Labhasetwar, 2003. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Bio.Mat., 55: 329-347.
Johann Plank, 2004. Applications of biopolymers in construction engineering. Bio Pol., 10: 31- 32.
Lam K.H., J.M. Schakenrad, H Esselbrugge, J Feijen, and P Nieuwenhuis, 1993. The effect of phagocytosis of poly (L-lactic acid) fragments on cellular morphology and viability. J Biomed Mater Res., 27: 1569-1577.
Middleton J.C. and J.A. Tipton, 1998. Synthetic biodegradable polymers as medical devices. Med. Pla. Biomat., 31 – 38.
Plank, and Johann, 2004. Application of Biopolymers and other Biotechnological Products in Building Material. App. Microbio. Biotech., 66: 1-9.
Van G, G Cornelis, and S Gayton, 1996. The Biodegradability and Non Toxicity of Carboxy Methyl Cellulose and Intermediates. Environ. Tox. Chem., 15, 270.
Weiler A, F.G. Hoffmann, C Stahelin, and Hanns-Joachim Helling, and P Sudkamp, 2000. Biodegradaable Implants in Sports Medicine: The Biological Base. Arthroscopy: The J. of Arthros. Rel. Sur., 16: 305–321.
Wolk S.K., G Swift, Y.H. Paik, K.M. Yocom, R.L. Smith, and E.S. Simon, 1994. Macromol., 27: 7613 – 7620.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









