{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Souvik Mukherjee*1, Deepronil Roy2 and Suman Das3
1Department of Pharmaceutical Sciences & Natural Products, Central University of Punjab, Bathinda, Punjab, India
2Department of Animal Sciences, Central University of Punjab, Bathinda, Punjab, India 3Department of Human Genetics & Molecular Medicine, Central University of Punjab, Bathinda, Punjab, India
mukherjees388@gmail.com
ABSTRACT
Malaria is a lifestyle-threatening tropical disorder, due to the intracellular parasite Plasmodium falciparum. The sector health employer counts malaria as one of the pinnacle ten reasons of worldwide demise. The unavailability of a successful malaria vaccine and the ever-increasing times of drug resistance in the malaria parasite call for the invention of new targets inside P. falciparum for the development of next generation antimalarial drug. Fortuitously, all apicomplexan parasites, along with P. falciparum harbor a relict, non-photosynthetic plastid referred to as the apicoplast. The apicoplast is a semi-self-sustaining organelle within P. falciparum, containing a 35 kb circular genome. Notwithstanding a genome of its own, majority of the apicoplast proteins are encoded by means of the parasite nucleus and imported into the apicoplast. The organelle has been proven to be vital to P. falciparum survival and the loss the apicoplast manifests as a ‘not on time loss of life’ response in the parasite. The apicoplast has advanced out of cyanobacteria in a complicated, two step endosymbiotic event. As a result, the architecture and the gene expression machinery of the apicoplast is pretty bacteria-like and is at risk of a wide variety of antibiotics consisting of fosmidomycin, tetracycline, azithromycin, clindamycin and triclosan. The biosynthetic pathways for isoprenoids, fatty acids and heme function within the malaria apicoplast, making the organelle a top notch goal for drug development. This review specializes in the evolution, biology and the essentiality of the apicoplast inside the malaria parasite and discusses a number of the current achievements toward the layout and discovery of apicoplast focused antimalarial drug.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2586
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 5 Received On: 20/03/2018; Accepted On: 06/04/2018; Published On: 01/05/2018 How to cite this article: Mukherjee S, Roy D, Das S; Apicoplast: a brilliant focus for antimalarial drug development; PharmaTutor; 2018; 6(5); 13-22; http://dx.doi.org/10.29161/PT.v6.i5.2018.13 |
INTRODUCTION
In old times, while human beings are regarding a lot extra at the life-style sickness, even in some of the international locations in which a massive range of population are stricken by old infectious diseases, malaria remains one of the predominant reasons of morbidity and mortality within the tropical and subtropical regions of the arena. Consistent with international malaria estimates inside the 12 months 2017, nearly 216 million human beings were on the danger and 445,000 deaths came about and most of them were P.falciparum cases. The emergence of resistant lines of malaria parasite (mainly P.falciparum) to almost all of the antimalarial capsules which might be currently in scientific use including chloroquine and sulphadoxine-pyrimethamine making it important to find out and develop each the brand new antimalarial tablets and remedy. Latest advances in molecular strategies at the side of the genome sequence of P.falciparum may also offer novel targets in metabolic pathways like isoprenoid biosynthesis, fatty acid biosynthesis and heme biosynthesis in the apicoplast of Plasmodium (Wu et al., 2015).Apicoplast, which is derived from an engulfed crimson alga also present in most of the apicomplexan parasites like plasmodium. Apicoplast homes prokaryotic equipment, to replicate the circular genome and to transcribe and translate the genes that it possesses. Because of its evolutionary distance from the host it is now considered as a vital organelle for the centered drug discovery (Burger, van Eijk, Bussink, Hill, & ter Kuile, 2016). The antibiotics which are inhibitors of the bacterial DNA gyrase inclusive of ciprofloxacin, inhibitors of translation inclusive of azithromycin, chloramphenicol, clindamycin, tetracycline and rifampicin results inside the so known as “not on time death impact” (Lim, Sayers, Goodman, & McFadden, 2016). Remedy of malaria with this antibiotics arrest the cellular growth within the subsequent asexual cycle however the parasites in the present day cycle remained exceptionally unaffected. Apart from those housekeeping processes apicoplast additionally offers the possibilities to have a look at extraordinary metabolic machineries together with isoprenoid, fatty acid and heme biosynthetic pathway collectively with their respective enzymes. The absence of a number of the enzymes concerned in those pathways in human host additionally makes them more appealing, novel and ideal targets (Visser et al., 2014).
A. MALARIA
Malaria is a protozoal sickness caused by the contamination with parasites of genus Plasmodium and female Anopheles mosquito. It’s far an acute infectious disease (Götz et al., 2017). When the mosquito bites it introduces the parasites into the man or woman’s blood from its saliva. The parasites then tour to the liver cells (hepatocytes) where they mature and reproduce. There are specifically 4 species of Plasmodium which can infect and be unfold through human beings such as Plasmodium vivax, P.falciparum, P.malariae and P.ovale. Amongst a lot of these species, majority of deaths are caused by Plasmodium falciparum due to the fact the opposite varieties of species generally reason a milder form of malaria. Malaria reasons signs like headache, fever with rigor, tiredness, vomiting, and profuse sweating and so on. The signs and symptoms of malaria usually start ten to 15 days after being bitten by using mosquito (Jackson &Black, 2017). Malaria is normally recognized through the microscopic exam of blood using blood films or every now and then with antigen based totally rapid diagnostic check.
B. MALARIA DISEASE BURDEN
According to WHO, African area continues to hold a disproportionately excessive proportion of the worldwide malaria burden (Thwing et al., 2017), greater than thirds (70%) of all malaria deaths arisen on this age organization. 3,000 kids beneath the age of five years die each day and about $12 billion GDP is misplaced in line with year because of expanded healthcare value, poor effects on tourism and lost ability to work (Alonso & Noor, 2017).
C. TYPES OF Plasmodium sp.
There are mainly four types of Plasmodium species which can cause malaria in human and they are listed in the table given below:
|
Plasmodium falciparum |
P. vivax |
P.malariae |
P.ovale |
|---|---|---|---|
|
They are the most dangerous species which can cause an acute and rapidly fulminating disease i.e., characterized by persistent high fever, Orthostatic Hypotension and Massive Erythrocytosis Capillary obstruction and death if treatment is delayed (Visser et al., 2014). |
It causes a milder form of the disease. |
It is common to many tropical regions of the World. |
It is rarely Encountered. |
Table 1: Types of Plasmodium species (Visser et al., 2014).
D. LIFE CYCLE OF MALARIA PARASITE
The malaria parasite lifestyles cycle entails two hosts, one is human and the opposite one is mosquito. For the duration of a blood meal, a malaria inflamed woman Anopheles mosquito inoculates sporozoites into the human host. The sporozoites then tour thru the blood movement and infect the liver cells (hepatocytes) where they mature into schizonts. The schizonts rupture and launch merozoites. After this initial replication within the liver cells which is referred to as the exo-erythrocytic schizogony, the parasites go through asexual multiplication within the erythrocytes called the erythrocytic schizogony. The merozoites which can be launched from the exo-erythrocytic stage infect the crimson blood cells and become ring level trophozoites (Brochet et al., 2014). This ring stage trophozoites mature into schizonts which rupture and launch merozoites. Some parasites differentiate into sexual erythrocytic levels called gametocytes. The male gametocytes are referred to as microgametocytes whilst the woman gametocytes are called macro gametocytes. These male and lady gametocytes are ingested by way of an Anopheles mosquito at some stage in blood meal. The parasite’s multiplication within the mosquito is referred to as the sporogonic cycle. Whilst within the mosquito’s stomach, the microgametes penetrate the macrogametes producing zygotes (Ke et al., 2015). With time the zygotes emerge as motile and elongated that are called ookinetes. Those ookinetes invade the midgut wall of the mosquito in which they turn into oocysts. The oocysts grow, rupture and release sporozoites which make their way to the mosquito’s salivary glands. Inoculation of the sporozoites into a brand new human host perpetuates the malaria life cycle (Siciliano & Alano, 2015).
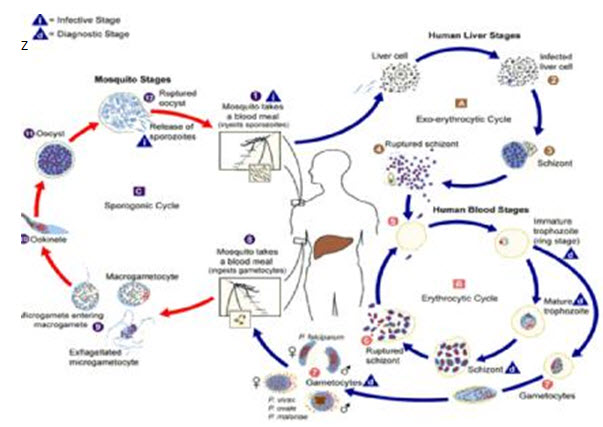
Fig 1: Life cycle of Plasmodium species (wcdc.gov)
E. MALARIA CONTROL STRATEGIES
Vector manipulate is the primary way to save you and reduce malaria transmission. If coverage of vector manipulate interventions inside a specific vicinity is high sufficient, then a degree of safety could be conferred across the network. Types of vector manage – insecticide-dealt with mosquito nets and indoor residual spraying – are powerful in a wide variety of circumstances. Antimalarial medicines also can be used to save you malaria. For guests, malaria can be averted thru chemoprophylaxis, which suppresses the blood level of malaria infections, thereby stopping malaria ailment (Bhatt et al., 2015). RTS, S/AS01 (RTS, S) – additionally known as Mosquirix – is an injectable vaccine that provides partial protection against malaria in young youngsters (Hemingway et al., 2016).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
F. OBJECTIVES AND USES OF ANTIMALARIAL DRUGS
The aims of using drugs in relation to malarial infection are:
1) To prevent clinical attack of malaria (Prophylactic).
2) To treat clinical attack of malaria (Clinical curative).
3) To completely eradicate the parasite from the patient’s body (Radical curative).
4) To cut down human to mosquito transmission.
These are achieved by attacking the parasite at its various stages of life cycle in the human host. Antimalarials that act on erythrocytic schizogony are called erythrocytic schizontocides, those that act on pre-erythrocytic as well as exo-erythrocytic (P. vivax) stages in liver are called tissue schizontocides, while those which kill gametocytes in blood are called gametocytes. Antimalarial drugs exhibit considerable stage selectivity of action. The classifications of antimalarial drugs according to their stage selectivity are given in the table below:
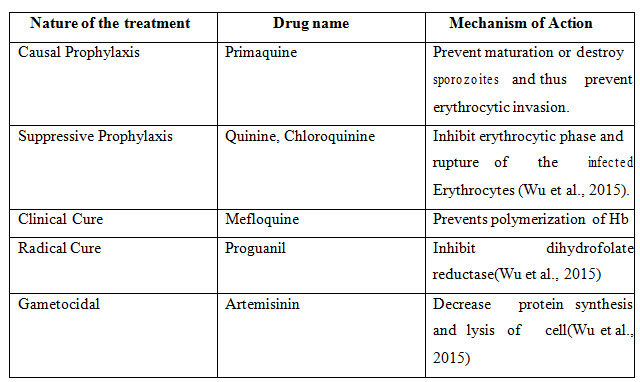
Table 2: Classification of Antimalarial drugs
G. COMBINATION THERAPY
In recent times the task in the control and remedy of malaria is the emergence, unfold and consolidation of parasite lines (mainly Plasmodium falciparum) which are resistant to conventional antimalarial pills which include chloroquine and sulphadoxine-pyrimethamine. Its miles said that in a few regions the Plasmodium falciparum strains are proof against artemisinin and its derivatives due to K13 propeller mutations (Phyto et al., 2016). the fear is if artemisinin resistant traces are unfold to the opposite parts of the world, artemisinin mixture therapy (ACT) which has been the simplest first-line remedy thus far in many nations may meet the identical destiny as the opposite tablets. That is why discovery and development of more modern, novel and lower priced antimalarial capsules and of latest remedies is a pressing necessicity.
Advantages of combination therapy over the other commonly used antimalarial drugs are:
1) Rapid clinical and parasitological cure.
2) High cure rates and low recrudescence rate.
3) Absence of parasite resistance (the components prevent development of resistance to each other).
4) Good tolerability profile (Price & White, 2018).
EXCELLENT TARGET FOR ANTIMALARIAL DRUGDEVELOPMENT
Plasmodium sp. are apicomplexan parasites and that they contain apicoplast which seems to serve its host with multiple feature. Several biosynthetic pathways are characterized inside the apicoplast. The parasites which can be cured of their apicoplast do no longer die right away but they lack the property of invasion and therefore fail to invade new host mobile (Chakraborty, 2016). This suggests that apicoplasts provide a few components which can be critical to invasion and or status quo of the parasitophorous vacuole in the host cell. Present day efforts to pick out these components propose that apicoplast includes numerous metabolic pathways consisting of isoprenoid, fatty acid and heme biosynthetic pathways (Uddin, McFadden, & Goodman, 2018). Unlike the antibiotics used inside the malaria treatment which reasons delayed dying, target to the enzymes worried in these anabolic pathways led to defying behind schedule dying impact in Plasmodium falciparum. The absence of some of the enzymes involved in these pathways in human host also makes them more appealing, novel and ideal objectives (Tesfaye, Prakash, & Singh, 2015).
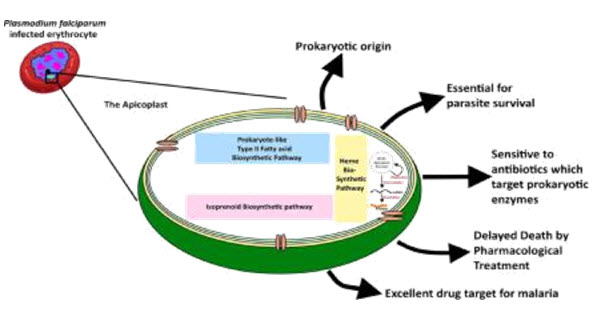
Fig 2: Excellent target for antimalarial drug development.
FATTY ACID BIOSYNTHESIS
Lipids are noticeably abundant components of all organisms which serve as constructing blocks of membranes, depots for energy garage and put up translational amendment which alter the localization and feature of protein. Fatty acid biosynthesis is critical for cellular boom, differentiation and homeostasis. Earlier than the discovery and characterization of various P.falciparum fatty-acid synthesis (FAS) enzymes, it changed into believed that P. falciparum lacks a de novo FAS pathway. However, this belief became rejected primarily based at the effects of the genomic sequencing of P. falciparum (Shears, Botté, & McFadden, 2015). The outcomes confirmed that P. falciparum is capable of synthesizing its own fatty acids. In addition, all of the enzymes of FAS pathway are recognized to be found in apicoplast. The FAS pathway present in the apicomplexan parasites are one of a kind from that in their human host or eukaryotic host. For example, in contrast to their eukaryotic hosts, the FAS pathway of apicomplexan parasites is catalyzed by way of distinct enzymes, and is referred to as typeII pathway or “dissociative” pathway. Then again, mammalian FAS pathway (type I or associative pathway) takes location in cytosol, and all steps of this pathway are catalyzed with the aid of fatty acid synthase, an unmarried giant polypeptide enzyme (Shears et al., 2017). FAS pathway in apicoplast is the principle source for the de novo synthesis of lipoic acid and fatty acid. Lipoic acid is synthesized each in apicoplast in addition to in mitochondria by way of the enzyme known as lipoic acid synthase. Lipoic acid is a robust antioxidant which might also have a main role in protecting the parasite in opposition to oxidative eight pressure in the course of the erythrocytic degree of its life cycle. The primary supply of carbon for this pathway is acetyl-CoA this is received either from acetate via the movement of acetyl-CoA synthetase or from pyruvate by pyruvate dehydrogenase complicated (PDHC). the primary enzyme in this pathway is acetyl CoA carboxylase (ACCase) which converts acetyl-CoA to malonyl-CoA that is later changed to malonyl-acetyl service protein (malonyl-ACP) by way of malonyl-CoA-ACP transacylase (MCAT). Β-ketoaceyl-ACP synthase III then catalyzes the condensation of malonyl-ACP with some other acetyl-CoA to shape a 3-oxoacyl-ACP product. Inside the elongation procedure, the carbonyl organization is eliminated and three-oxoacyl-ACP is reduced to 3-hydroxyacyl-ACP by 3-oxoacyl-ACP reductase (OAR) that enters in dehydration cycle (Oriero, Van Geertruyden, Jacobs, D'Alessandro, &Nwakanma, 2015). As soon as produced, the enzyme β-hydroxy-acyl-ACP dehydratase converts three-hydroxyacyl-ACP into enoyl-ACP which enters into the second reductive step (catalyzed through enoyl-ACP reductase) to complete the primary round of elongation cycle.
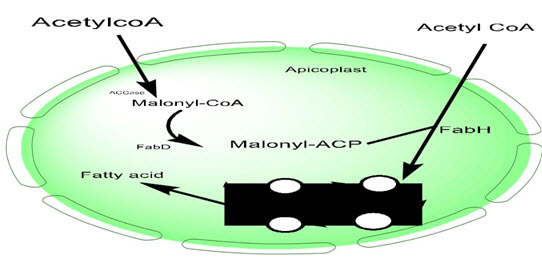
Fig 3: Fatty acid biosynthesis pathway.
1 INHIBITORS OF FATTY ACID BIOSYNTHESIS
Several research on FAS II have pronounced that the growth of the blood-stage of Plasmodium may be inhibited with the aid of blocking various enzymes. it's been confirmed that the deletion of the vital enzymes of FAS II that are Fab B/F and Fab Z, did now not cause any effect on asexual blood stage replication but the overdue liver degree of such parasites changed into unable to shape exo-erythrocytic merozoites (Schoors et al., 2015).Some of the inhibitors which can inhibit fatty acid biosynthesis are given in the table below:

Table 3: Fatty acid biosynthesis inhibitors.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
HEME BIOSYNTHESIS
Besides detoxifying the heme that comes from the host hemoglobin degradation, Plasmodium falciparum also synthesizes its very own heme. Heme is a vital prosthetic institution in various proteins which includes cytochromes. It’s been showed that the heme biosynthetic pathway found in P. falciparum is shared amongst apicoplast, mitochondrion and cytosol of the parasite. Because there's an overlap between the mammalian and the parasitic enzymes involved inside the heme biosynthetic pathway, those enzymes which can be of endosymbiosis origin are thought to be expedient to target (Wiley et al., 2015). Heme synthesis pathway in the mitochondria of malaria parasite starts with the synthesis of aminolaevulinic acid (δ-ALA) from glycine and succinyl-CoA that is catalyzed by ALA synthase. After the synthesis of ALA in the mitochondrion, heme biosynthetic pathway relocates itself to the apicoplast which contains enzymes like, δ-aminolaevulinate dehydratase (Hem B), porphobilinogen deaminase (Hem C) and uroporphyrinogen III decarboxylase (Hem E). Ferrochelatase (Hem H) is the closing enzyme inside the heme biosynthetic pathway which is positioned within the mitochondrion (Mailu et al., 2015). It’s far liable for insertion of ferrous iron into the protoporpherin IX
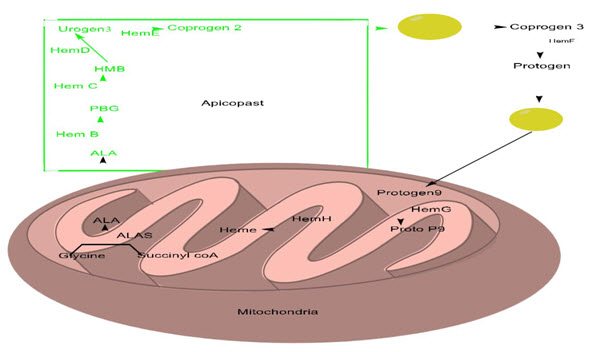
Fig 4: Heme biosynthesis pathway.
B.1 INHIBITORS OF HEME BIOSYNTHESIS
There are different types of inhibitors of heme biosynthesis are discovered till now. Some of these inhibitors along with their mechanism of action is given in the table below
| Compound name | Mechanism of action |
|---|---|
| Oxyfluorfen | It is a potent inhibitor of protoporphyrinogen oxidase which membrane bound enzyme involved in the heme and chlorophyll pathway (Tesfaye et 2015). |
| Succinyl acetone | It inhibits ALAD (Tesfaye et al., 2015). |
| Atovaqunone | It blocks the mitochondrial electron transport at complex III of the respiratory chain of protozoa and thereby inhibiting pyrimidine synthesis thus preventing DNA synthesis and leading to protozoal death (Tesfaye et al., 2015). |
| Aclonifen | It blocks the mitochondrial electron transport chain at complex III of the respiratory chain of protozoa (Tesfaye et al., 2015). |
Table 4: Inhibitors of heme biosynthesis
C. ISOPRENOID BIOSYNTHESIS
Isoprenoid biosynthesis pathway is one of the pathways in malaria parasite which comprise enzymes which can be determined in multiple compartment to supply its final merchandise i.e. isoprenoids. Isoprenoids are the various variety of compounds which are assembled from two common 5-carbon precursors referred to as isopentenyl pyrophosphate (IPP) and dimethyl-allyl diphosphate (DMAPP) (Jomaa et al., 1999). Some of the normally recognized isoprenoids are (i) steroids which can be worried inside the membrane structure, (ii) heme A and ubiquinone which might be believed to have significant contribution in the electron delivery, (iii) dolichol that is required for glycoprotein synthesis, and (iv) isopentyladenine that is found in some tRNAs. In mammals, isoprene units are acquired from the standard mevalonate pathway. In apicomplexa, the methyl erythritol phosphate (MEP) pathway or 1-deoxy-D-xylulose 5-phosphate (DOXP) pathway produces IPP and DMAPP. This pathway depends on the condensation of 3 molecules of acetyl CoA (coenzyme A) into HMG-CoA (3-hydroxy-three-methylglutaryl coenzyme A) that is reduced to mevalonate by using HMG-CoA reductase. Mevalonate is then transformed into IPP with mevalonate 5-phosphate as an intermediate. An important difference between cytoplasmic acetate/mevalonate pathway and the plastid DOXP pathway is that the previous uses starting compounds of two-carbon constructing blocks but the latter uses each -carbon in addition to three-carbon constructing blocks (Ralph, D'Ombrain, & McFadden, 2001). In apicoplast, triose phosphate importers along with the following enhancing enzymes generate pyruvate alongside glyceraldehydes-3-phosphate (GA3P) that are used by the first enzyme of the DOXP pathway, DOXP synthase (DXS). DXS condenses pyruvate and GA3P to DOXP. DOXP reductoisomerase (DXR) is then catalyzes the intermolecular rearrangement and reduction of DOXP to MEP. Subsequent reactions which contain different enzymes convert MEP to IPP and DMAPP which can be used as an intermediate in the production of isoprenoids which include dolichol and ubiquinones. After their cargo to cytoplasm, IPP and DMAPP are then condensed to GPP (geranyl diphosphate) by means of the enzyme FPPS (farnesyl diphosphate synthase). GPP is then condensed with the second one molecule of IPP to form FPP (farnesyl diphosphate). Sooner or later, FPP is transformed to GGPP (geranylgeranyl diphosphate) via GGPPS (geranylgeranyl diphosphate synthase) (Goodman, Su, & McFadden, 2007).

Fig 5: Isoprenoid biosynthesis pathway.
C.1 INHIBITORS OF ISOPRENOID BIOSYNTHESIS
Drugs involved in the inhibition of the malarial parasite’s isoprenoid biosynthesis are given in the table below:

Table 5: Isoprenoid biosynthesis inhibitors.
RESEARCH PIPELINE DRUGS
The name of the drugs which can be used as combination therapy against malaria are listed in the table given below:
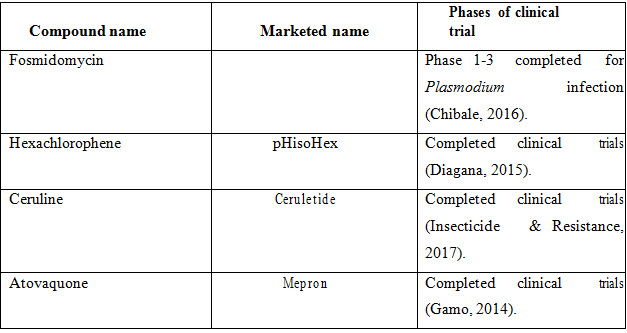
Table 6: Research pipeline drugs.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
FUTURE PROSPECTS
Mixture remedy is the contemporary desire to retard and roll-back the resistance disaster and might be the most effective alternative to be had to increase the working lifestyles of medication which can be at the front-line within the warfare against malaria. Aside from delaying the emergence of resistance, it is a bare fact that a mixture of two tablets that act on distinct goals (enzymes) in the equal metabolic pathway may additionally give correct efficacy and toxicological profiles. The search for the goals to discover new antimalarial drug has been made by way of the identity of recent capability objectives in apicoplastic metabolic pathways, which might be recognized to be evolutionarily distant from the ones of the human beings. Following this a number acknowledged and new lead compounds were screened for his or her inhibitory interest in opposition to metabolic enzymes like DXR, DXS and FPPS in isoprenoid pathway; ENR, the KAS family, KAR and HAD in FAS II; and ALAD and PPO in heme synthesis pathways. The current advances within the in vitro antimalarial screening assays by way of the usage of the entire parasites, we can10 desire that there stays a lot to be completed inside the pursuit of new mixtures of medicine which target distinct enzymes belonging to at least one unique metabolic pathway. Apicoplast is essential for growth and survival of the malaria parasite and has been notably centered. It houses vital biosynthetic pathways as well as prokaryotic like house responsibilities techniques presenting appealing avenues for drug improvement. The discovery of the susceptibility of the apicoplast to antimicrobial antibiotics has pushed giant research into the discovery of novel apicoplast targeting compounds. The apicoplast metabolic methods especially the prokaryote like fatty acid biosynthetic pathway has been drastically targeted over the latest years using medicinal chemistry and SAR based totally procedures.
REFERENCES
1. Alonso, P., & Noor, A. M. The global fight against malaria is at crossroads. The Lancet, 2017, 390 (10112), 2532-2534.
2. Bhatt, S., Weiss, D., Cameron, E., Bisanzio, D., Mappin, B., Dalrymple, U., Eckhoff, P, The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526(7572), 207.
3. Brochet, M., Collins, M. O., Smith, T. K., Thompson, E., Sebastian, S., Volkmann, K., Berriman, M. Phosphoinositide metabolism links cGMP-dependent protein kinase G to essential Ca2+ signals at key decision points in the life cycle of malaria parasites. PLoS biology, 2014, 12(3), e1001806.
4. Burger, R. J., van Eijk, A. M., Bussink, M., Hill, J., & ter Kuile, F. O. Artemisinin-based combination therapy versus quinine or other combinations for treatment of uncomplicated Plasmodium falciparum malaria in the second and third trimester of pregnancy: a systematic review and meta-analysis. Paper presented at the Open forum infectious diseases, 2016
5. Chakraborty, A. . Understanding the biology of the Plasmodium falciparum apicoplast; an excellent target for antimalarial drug development. Life sciences, 2016, 158, 104-110.
6. Chibale, K. , How Africa is helping expand the global antimalarial drug pipeline: health research, 2016, Quest, 12(3), 22-23.
7. Diagana, T. T. Supporting malaria elimination with 21st century antimalarial agent drug discovery. Drug discovery today, 2015, 20(10), 1265-1270.
8. Gamo, F.-J. Antimalarial drug resistance: new treatments options for Plasmodium. Drug Discovery Today: Technologies, 2014, 11, 81-88.
9. Goodman, C. D., Su, V., & McFadden, G. I. The effects of anti-bacterials on the malaria parasite Plasmodium falciparum. Molecular and biochemical parasitology, 2007, 152(2), 181-191.
10. Götz, A., Ty, M., Chora, A. F., Zuzarte-Luís, V., Mota, M. M., & Rodriguez, A. Innate Immunity to Malaria Malaria 2017, (pp. 3-25): Springer.
11. Hemingway, J., Shretta, R., Wells, T. N., Bell, D., Djimdé, A. A., Achee, N., & Qi, G. Tools and strategies for malaria control and elimination: what do we need to achieve a grand convergence in malaria? PLoS biology, 2016, 14(3), e1002380.
12. Insecticide, m. R. C. P. o., & Resistance, D. malERA: An updated research agenda for insecticide and drug resistance in malaria elimination and eradication. PLoS medicine, 2017, 14(11), e1002450.
13. Jackson, B. D., & Black, R. E. (2017). A literature review of the effect of malaria on stunting. The Journal of nutrition, 2017, 147(11), 2163S-2168S.
14. Jomaa, H., Wiesner, J., Sanderbrand, S., Altincicek, B., Weidemeyer, C., Hintz, M., . . .
15. Lichtenthaler, H. K. Inhibitors of the nonmevalonate pathway of isoprenoid biosynthesis as antimalarial drugs. Science, 1999, 285(5433), 1573-1576.
16. Ke, H., Lewis, I. A., Morrisey, J. M., McLean, K. J., Ganesan, S. M., Painter, H. J., . . . Vaidya, A. B. Genetic investigation of tricarboxylic acid metabolism during the Plasmodium falciparum life cycle. Cell reports, 2015, 11(1), 164-174.
17. Lim, L., Sayers, C. P., Goodman, C. D., & McFadden, G. I. (2016). Targeting of a transporter to the outer apicoplast membrane in the human malaria parasite Plasmodium falciparum. PloS one, 2016, 11(7), e0159603.
18. Siciliano, G., & Alano, P.. Enlightening the malaria parasite life cycle: bioluminescent Plasmodium in fundamental and applied research. Frontiers in microbiology, 6, 391.
19. Tesfaye, S., Prakash, B., & Singh, P. P. . Apicoplast Biosynthetic Pathways as Possible Targets for Combination Therapy of Malaria. Journal of Pharmacy and Pharmacology, 2015, 3(1), 101-115.
20. Thwing, J., Eckert, E., Dione, D. A., Tine, R., Faye, A., Yé, Y., Diouf, M. B. Declines in Malaria Burden and All-Cause Child Mortality following Increases in Control Interventions in Senegal, 2005–2010. The American journal of tropical medicine and hygiene, 2017, 97(3_Suppl), 89-98.
21. Uddin, T., McFadden, G. I., & Goodman, C. D. Validation of Putative Apicoplast-Targeting Drugs Using a Chemical Supplementation Assay in Cultured Human Malaria Parasites. Antimicrobial agents and chemotherapy, 2018, 62(1), e01161-01117.
22. Visser, B. J., Wieten, R. W., Kroon, D., Nagel, I. M., Bélard, S., van Vugt, M., & Grobusch, M. P. Efficacy and safety of artemisinin combination therapy (ACT) for non-falciparum malaria: a systematic review. Malaria journal, 2014, 13(1), 463.
23. Wiley, J. D., Merino, E. F., Krai, P. M., McLean, K. J., Tripathi, A. K., Vega-Rodríguez, J., Cassera, M. B. Isoprenoid precursor biosynthesis is the essential metabolic role of the apicoplast during gametocytogenesis in Plasmodium falciparum. Eukaryotic cell, 2015, 14(2), 128-139.
24. Wu, W., Herrera, Z., Ebert, D., Baska, K., Cho, S. H., DeRisi, J. L., & Yeh, E. A chemical rescue screen identifies a Plasmodium falciparum apicoplast inhibitor targeting MEP isoprenoid precursor biosynthesis. Antimicrobial agents and chemotherapy, 2015, 59(1), 356-364.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









