{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Garima shrivastava*, Dr.Manjul shrivastava, Gaurav shrivastava
Department of Chemistry,
Govt.M.H. College of Home Science,
Jabalpur, MP, India
ABSTRACT
In an attempt to find out a new class of antimicrobial agents new schiff base ligand of 2-amino 5-nitro thiazole and its copper complex were prepared. The Microwave assisted synthesis was carried out by condensation of 2-amino 5-nitro thiazole and substituted salicyldehyde. The newly synthesized schiff base ligand and its copper complex were tested against representatives of gram-positive (Staphylococcus aureus, Bacillus subtilis) and gram- negative bacteria (E. coli, Pseudomonas putida) and fungi (Candida albicans, Aspergillus niger) by disc diffusion method.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2608
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 9 Received On: 12/05/2018; Accepted On: 08/08/2018; Published On: 01/09/2018 How to cite this article: Shrivastava, M., Shrivastava, G. and Shrivastava, G. 2018. Antimicrobial activity of Schiff Base of 2-Amino 5-Nitrothiazole and its Copper complex. PharmaTutor. 6, 9 (Sep. 2018), 1-5. DOI:https://doi.org/10.29161/PT.v6.i9.2018.1 |
INTRODUCTION
Microwave heating technology is green chemical process , the application of microwave-assisted is useful technology in drug synthesis because it is simple, sensitive, reducing the hazard, often possible to reduce reaction times to a few minutes under solvent free or lower solvent and increase the yields and easier work up as compared to conventional methods(Ahmed Hassen et al.,2016).Those compounds which containing imine group (-HC=N-) are known as Schiff bases, which were first reported by Hugo Schiff in 1864 and formed by condensation of a primary amine with an active carbonyl compound, and generally take place under acid, base catalysis or with heat(Gadada Naganagowda.,2014). Heterocyclic ring -containing Schiff bases can show naturally increased biological activities.
The literature survey reveals that the thiazole ring is present in various natural compounds which shows useful biological activities. It occurs for example, in thiamine, a coenzyme is required for the oxidative decarboxylation of α-keto acids. A tetra hydrothiazole also appears in the skeleton of penicillin, which is one of the first and still most important broad-spectrum antibiotics.
Thiazole, thiazol amines are valuable medicines (Dash B,1981; Metzger J V.,1979; Troutman H D. ,1948;). It is obvious that compounds with the thiazole ring have a potential biological activity. The thiazole derivatives gained more importance and interest due to their anticancer (Ahmed R. Ali.,2014; Funda Tay.,2017; Sobhi M. Gomha,2017;), anticonvulsant (Faiz Arshad,2014; Nour E. A. Abdel-Sattar,2017), antitumor (S. Muralikrishna,2013; Wen-Xi Cai ,2016;) antiviral (Nuray Ulusoy Guzeldemirci,2017;)and fungicidal activities (Khalid Mohammed Khan,2012;). They are also used as, anti-inflammatory (Firas A. Hassan,2012;) and anthelmintic agents (Amit S. Lunkad.,2013; Balaji P. N.,2014;) agents. Metal ions play a vital role in a vast number of different biological processes through co-enzymatic systems. The interaction of these ions with biologically active ligands, for instance in drugs, is a subject of great interest. Some biologically energetic compounds act via chelation, but for most of them little is known about how metal coordination influences their activity.
We report here the Microwave assisted synthesis and antimicrobial evaluation of schiff base ligand 2,4-diiodo-6-{(E)-[(5-nitro-1,3-thiazol-2-yl) imino] methyl} phenol and its copper complex incorporating both thiazole moiety and substituted salicyldehyde through imine linkages at 2-position of thiazole.
MATERIAL AND METHODS
Microwave assisted Synthesis of schiff base ligand
2-amino 5-nitro thiazole (0.01 mol) and 3,5-diiodosalicyldehyde (0.01 mol), and were mixed thoroughly in ethanol and small amount of glacial acetic acid was added and were taken in Erlen Meyer flask capped with a funnel placed in a microwave oven and irradiated an interval of 1 min at 450W for about 5-8 min. After completion the reaction, the reaction mixture was allowed to attain room temperature and solid separated was filtered. The resulting product was then recrystallized with ethanol, finally dried under reduced pressure over anhydrous CaCl2 in a desiccator. Yield: 70-90%.
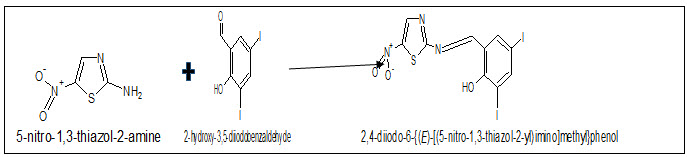
Scheme-1, Microwave assisted synthesis of schiff base
EXPERIMENTAL
ANTIMICROBIAL ACTIVITY
The newly synthesized schiff base ligand 2,4-diiodo-6-{(E)-[(5-nitro-1,3-thiazol-2-yl) imino] methyl} phenol and its copper complex were evaluated for in vitro bacterial activity against as example of Gram-positive bacteria (staphylococcus aureus and Bacillus Subtilis) and gram negative bacteria (E. coli, Pseudomonas putida) by disc diffusion method was for the determination of the preliminary antibacterial activity.
The bacterial cultures were incubated at 30 ± 0.1 0C for 24 hours by inoculation into nutrient agar. The compounds were dissolved in test solution (DMSO) and the disc of Whatman filter paper No. 41 having the diameter 4mm were prepared and soaked in it. These soaked discs were placed on nutrient agar plates inoculated with bacteria. These plates were incubated for 36 hrs. At 30 0C. The inhibition zone was observed after 36 hr.
Anti-fungal screening: The fungal strains were directly mixed with the PDA medium (potato dextrose agar) and dispersed into the petri plates. Filter paper discs of 4 mm diameter were prepared prior to the experiment. These discs were soaked in DMSO in which the schiff base and its copper complex were dissolved and DMSO was used as a control. The filter paper discs were placed on nutrient medium mixed with fungal strains. These Petri dishes were incubated at 350C for 48 hrs. The growth of the fungus was measured by recording the diameter of fungal mycelia.
The result was recorded for schiff base and its copper complex as the average diameter of inhibition zones (IZ) of bacterial growth around the discs in mm. The antibacterial activity of schiff base and their copper complex in DMSO against of Gram-positive bacteria (staphylococcus aureus and Bacillus subtilis) and gram negative bacteria (E. coli, Pseudomonas putida) .is shown inTable-1 and fugal activity results shown in Table-2.
RESUTS AND DISCUSSION
Antibacterial activity results, Table 1 showed that free Schiff base ligand showed no activity against bacterial strains. The schiff base Cu(II) complex exhibited moderate activity against S. aureus, and E. coli and had a higher effect against Bacillus subtilis but no activity against Pseudomonas putida microorganism. The results of antifungal activities of synthesized complex are summarized in table 2. The in vitro antifungal activity of metal complex showed very good inhibition than the schiff’s base ligand against fungal strains under investigation.
Antibacterial and antifungal activities of the schiff base ligand and its copper complex were compared with those of the standard drugs ampicillin and fluconazole. The discrepancy in the activity of copper complex against different microorganisms depends on the permeability of the cell or differences in the ribosomes in the microbial cells. (S. K. Sengupta,1998;) The lipid membrane surrounding the cell of microorganism favours the passage of any lipid soluble materials and it is known that liposolubility is an important factor controlling antimicrobial activity (J. Parekh,2005; Y. Vaghasia,2004;). It was evident from the data that in vitro significantly increased on coordination. Moreover, coordination reduces the polarity (B.C.J. Bayer ,1962; R. Pal, 2014;) of the metal ion mainly because of the partial sharing of its positive charge with the donor groups (A.B.P. Lever, 1984) within the chelate ring system formed. This process, in turn, leads to increase in the lipophilic character of metal chelate so as to make it more permeable through the lipid layer of microorganism (Chohan ZH, U.M. Hassan, 2002;) thus destroying them more aggressively.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table -1: Antibacterial Activity of the Schiff’s base Ligand and its complex
|
|
Zone of Inhibition (diameter in mm) |
|||||
|
S.No |
Compound code |
E.coli |
S. Aureus |
Pseudomonas putida |
B. Subtilis |
|
|
1 |
ATDI-S |
- |
- |
- |
- |
|
|
2 |
ATDI-C |
- |
15 |
- |
26.5 |
|
|
3 |
Positive Control |
40 |
24 |
34 |
46 |
|
Table-2: Antifungal activity of schiff base ligand and its copper complex
|
|
Zone of Inhibition (diameter in mm) |
|||
|
S.NO |
Compound Code |
Candida albicans |
Aspergillus niger |
|
|
1 |
ATDI-S |
11 |
- |
|
|
2 |
ATDI-C |
20 |
19 |
|
|
3 |
Positive Control |
24 |
25 |
|
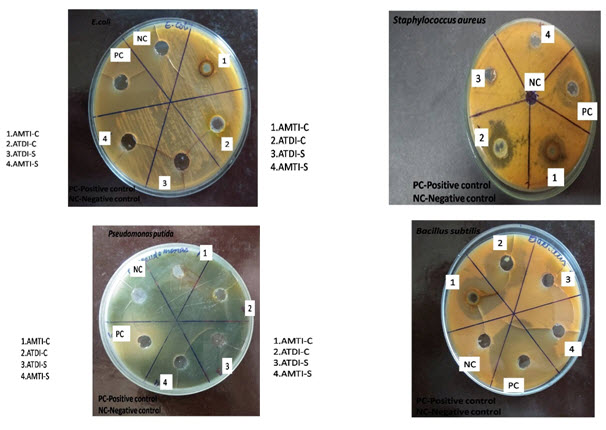
Fig-1 Antibacterial activity of schiff base and its copper complex
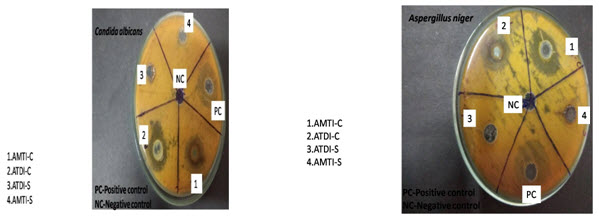
Fig-2 Antifungal activity of schiff base ligand and copper complex
CONCLUSION
In present study we reported a nonconventional microwave assisted synthesis of schiff base ligand 2,4-diiodo-6-{(E)-[(5-nitro-1,3-thiazol-2-yl) imino] methyl} and their copper complex. In vitro antimicrobial activity of copper complex and ligand were studied and it has been observed that copper complex of schiff base were found to display higher antibacterial activity than free schiff base ligand under identical experimental condition. Some structural modification in free schiff base ligand (2,4-diiodo-6-{(E)-[(5-nitro-1,3-thiazol-2-yl) imino] methyl}) can tune the antimicrobial activity and may serve as a model compound for the development of some efficient antimicrobial activity.
REFERENCES
1. A.B.P. Lever,1984, Inorganic electronic spectroscopy Elsevier, Amsterdam 1984.
2. Ahmed Hassen Shntaif, Zahraa M. Rashid, (2016); The Synthesis of Schiff bases under microwave Irradiation: Review, Journal of Chemical and Pharmaceutical Science, 9 (3) ; 1066 - 1068
3. Ahmed R. Ali, Eman R. El-Bendary, Mariam A. Ghaly, Ihsan A. Shehata,2014. Synthesis, in vitro anticancer evaluation and in silico studies of novel imidazo[2,1-b] thiazole derivatives bearing pyrazole moieties, European Journal of Medicinal Chemistry 75 ,492-500.
4. Amit S. Lunkad, Sachin N. Kothawade, Krishna K. Darkunde, Bhanu Priya, Ujwala R. Bagmar and Dipali S. Bhandari, 2013Synthesis and Screening Antihelminitic Activity of Some Thiazole Derivatives, Int. J. Chem. Sci.: 11(2),1146-1152.
5. B.C.J. Bayer,1962, An introduction to ligand field. McGraw Hill, New York 1962.
6. Balaji P. N., N. Chanukya, A. Keerthi, Mohana. C, K. Hima Bindu and S. Priyanka,2014. In vitro anthelmintic and antimicrobial activity of synthesized fluoro & nitro substituted N-[(Z)-phenylmethylidene]-1,3-benzothiazol-2- amine and its derivatives, Pelagia Research Library der Chemica Sinica, 5(1):110-114.
7. Chohan ZH, Scozzafava A, Supuran CT (2002); Unsymmetrical 1,1-disubstituted ferrocenes: synthesis of Co(II), Cu(II), Ni(II) and Zn(II) chelates of ferrocenyl-1-thiadiazolo-1- tetrazole-1-thiadiazolo-1-triazole and -1-tetrazolo-1-triazole with antimicrobial properties. J Enzym Inhib Med Chem, 2002; 17(4): 261–266.
8. Dash B, Praharaj S and Mohapatra. 1981.P K, J Indian Chem Soc., 58,1184.
9. Faiz Arshad, Nadeem Siddiqui, Ahmed Elkerdasy, Abdulmohsen H. Al Rohaimi and Suroor A. Khan,2014. Anticonvulsant and Neurotoxicity Evaluation of Some Newly Synthesized Thiazolyl Coumarin Derivative, American Journal of Pharmacology and Toxicology 9 (2): 132-138,
10.Firas A. Hassan, (2012) ; Synthesis, Characterization, Anti-inflammatory, and Antioxidant Activities of Some New Thiazole Derivatives, International Journal of Applied Science and Technology, 2 (7); 180-187
11.Funda Tay, Cell Erkan, Nalan Yilmaz Sariozlu ,2017. Emel, Seref Demirayak, Synthesis, antimicrobial and anticancer activities of some naphthylthiazolylamine derivatives, Biomedical Research. 28 (6): 2696-2703.
12.Gadada Naganagowda, Reinout Meijboom, and Amorn Petsom (2014) ; Synthesis and Antimicrobial Activity of New Schiff Base Compounds Containing 2-Hydroxy-4-pentadecylbenzaldehyde Moiety, Hindawi Publishing Corporation Advances in Chemistry.
13.Hassan UM, Chohan ZH, Supuran CT. (2002); Antibacterial Zn(II) compounds of Schiff base derived from some benzothiazoles. Main Group Met Chem, 25(5) : 291-296.
14.J. Parekh, P. Inamdhar, R. Nair, S. Baluja, S. Chandra.2005, Synthesis and antibacterial activity of some Schiff bases derived from 4-aminobenzoic acid.J. Serb. Chem. Soc. 70 (10), 1155–1161.
15.Khalid Mohammed Khan, Nida Ambreen, Aneela Karim, Sumayya Saied, Afroze Amyn, Aqeel Ahmed, and Shahnaz Perveen, 2012Schiff Bases of Thiazole as Antibacterial and Antifungal Agents, Journal of Pharmacy Research ,5(1),651-656.
16.Metzger J V, (1979) ; The Chemistry of Heterocyclic Compounds, A Series of Monographs, Thiazole and Its Derivatives, Wiley, Chichester.
17.Nour E. A. Abdel-Sattar, Abeer M. El-Naggar, and M. S. A. Abdel-Mottaleb,2017. Novel Thiazole Derivatives of Medicinal Potential: Synthesis and Modeling, Hindawi Journal of Chemistry, Article ID 4102796.
18.Nuray Ulusoy Guzeldemirci, Berin Karaman, Ömer Kucukbasmaci,2017 .Antibacterial, Antitubercular and Antiviral Activity Evaluations of Some Arylidenehydrazide Derivatives Bearing Imidazo[2,1-b] thiazole Moiety, Turk J Pharm Sci .14(2):157-163.
19.R. Pal, V. Kumar, A.K. Gupta, V. Hassan UM, Chohan ZH, Supuran CT.(2002) ; Antibacterial Zn(II) compounds of Schiff base derived from some benzothiazoles. Main Group Met Chem, 25(5) : 291-296.
20.S. K. Sengupta, O. P. Pandey, B. K. Srivastava, V. K. Sharma, Synthesis, structural and biochemical aspects of titanocene and zirconocene chelates of acetylferrocenyl thiosemicarbazones,1998 Transition Met. Chem. 23(4), 349.-353.
21.S. Muralikrishna, P. Raveendrareddy, L. K. Ravindranath, S. Harikrishna and P. Jagadeeswara Rao,2013. Synthesis Characterization and antitumor activity of thiazole derivatives containing indole moiety bearing-tetrazole, Der Pharma Chemica,5(6):87-93.
22.Sobhi M. Gomha, Nabila A. Kheder, Mohamad R. Abdelaziz, Yahia N. Mabkhot, Ahmad M. Alhajoj, Gomha et al (2017) ; a facile synthesis and anticancer activity of some novel thiazoles carrying 1,3,4-thiadiazole moiety, . Chemistry Central Journal 11:25.
23.Troutman H D and Long L M,1948. J Am Chem Soc., 70, 3436-3439.
24.Wen-Xi Cai, Ai-Lin Liu, Zheng-Ming Li, Wei-Li Dong, Xing-Hai Liu, and Na-Bo Sun ,(2016) Synthesis and Anticancer Activity of Novel Thiazole-5-Carboxamide Derivatives, Appl. Sci. 6, (8) :1-10
25. Y. Vaghasia, R. Nair, M. Soni, S. Baluja, S. Chandra,2004, Synthesis, structural determination and antibacterial activity of compounds derived from vanillin and 4-aminoantipyrineJ. Serb. Chem. Soc. 69 (12) ;-998.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









