{ DOWNLOAD AS PDF }
ABOUT AUTHORS
S. Dhivya*, DR. A. N. Rajalakshmi
Department of Pharmaceutics, Mother Theresa Post Graduate and Research Institute of Health Sciences, Gorimedu, Puducherry-605006, India.
ABSTRACT:
Design and development of herbal nanoparticles has become a frontier research in the nanoformulation arena. Curcumin is the main bioactive component contained in Curcuma longa, largely employed in traditional medicine. Recently, beneficial properties, useful for prevention and treatment of several disorders, have been discovered for this compound. Although curcumin has shown therapeutic efficacy against many human ailments, one of the major problems with curcumin is its poor bioavailability, which appears to be primarily due to poor absorption, rapid metabolism, and rapid systemic elimination. Therefore introduction of nanotechnology provides a solution towards increased bioavailability of curcumin. In this review, the various methods of preparation of curcumin nanoparticles are briefly discussed.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2604
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 9 Received On: 01/06/2018; Accepted On: 27/07/2018; Published On: 01/09/2018 How to cite this article: Rajalakshmi, N. and Dhivya, S. 2018. A Review on the preparation methods of Curcumin Nanoparticles. PharmaTutor. 6, 9 (Sep. 2018), 6-10. DOI:https://doi.org/10.29161/PT.v6.i9.2018.6 |
INTRODUCTION:
Curcumin (curcumin-I) (Masuda et al., 1992) is the active ingredient of the dietary spice
turmeric (Curcuma longa, Family-Zingiberaceae) and has been consumed for medicinal purposes for thousands of years. The other curcuminoids present in turmeric are demethoxycurcumin (curcumin-II) (Sreejayan et al., 1994), bisdemethoxycurcumin (curcumin-III) (Unnikrishnan et al., 1995).
Curcumin has wide range of applications such as anti-bacterial activity, anti-inflammatory, anti-oxidant, pro-apoptotic, chemopreventive, chemotherapeutic, anti-proliferative, wound healing, anti-nociceptive, antiparasitic, anti-malarial, diabetes, obesity, neurologic, psychiatric disorders and cancer, as well as chronic illnesses affecting the eyes, lungs, liver, kidneys, and gastrointestinal and cardiovascular systems (Prasad et al., 2014; Anand et al., 2008). Although curcumin has shown therapeutic efficacy against many human ailments, one of the major problems with curcumin is its poor bioavailability (Anand et al., 2007) which appears to be primarily due to poor absorption, rapid metabolism, and rapid systemic elimination. Therefore, efforts have been made to improve curcumin's bioavailability by improving these features. The promising approaches to increase the bioavailability of curcumin include the use of nanoparticles (Tiyaboonchai et al., 2007), liposomes (Li et al., 2005), micelles (Suresh et al., 2007), phospholipid complexes (Liu et al., 2006), and structural analogues (Preetha et al., 2007; Ohori et al ., 2006). With the ongoing development of nanotechnology, the nanoparticles have its own importance in novel drug delivery system. The application of nanoparticle formulation will deliver new approaches for enhancement of solubility, stability, bioavailability and pharmacological activity and ability to avoid physical and chemical degradation. Therefore introduction of nanotechnology in curcumin provides a solution towards increased bioavailability and therapeutic efficacy.
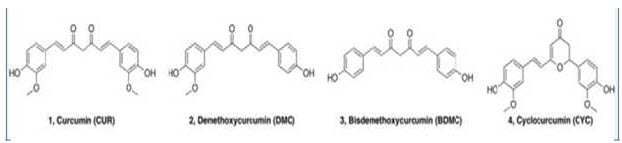
Chemical Structure of curcuminoids
Methods of preparation:
• Coacervation techniques
• Nanoprecipitation method
• Spray drying method
• Single emulsion method
• Solvent evaporation method
• Microemulsion
• Wet milling method
• Thin film hydration method
• Solid dispersion method
• Emulsion polymerization method
• Fessi method
• Ionic gelation method
• Ultrasonication
• Antisolvent precipitation method
Coacervation techniques: In this method of synthesis, the polymer is dissolved in organic solvent (e.g. dichloromethane, ethyl acetate, or acetonitrile) and herbal drug (curcumin) is suspended directly in polymeric solution and it is allowed to homogenize properly. Nanoparticles are collected by centrifugation. It is inexpensive method. The main drawback of this method is that it requires large amount of solvent. Chirio et al., 2011 formulated curcumin loaded nanoparticles by using this technique.
Nanoprecipitation method: Nanoprecipitation method is also known as Solvent displacement method. In this method, desired polymer is suspended in the suitable solvent to form polymeric solution and herbal drug (curcumin) is added into it. After that this drug- polymer solution is added into water under continuous stirring which results in precipitation. After that the solvent is allowed to evaporate by hot air flow. Spray drying resulted in the formation of drugs in the amorphous state, which may get partially crystallized during processing. In this method of synthesis, curcumin and polymer are dissolved in same solvent or mixture of solvents. Chin et al., 2014 prepared starch nanoparticles for controlled release of curcumin.
Spray drying method: Curcumin nano-crystals can be formulated by spray drying method. For that Curcumin nano-suspensions, having a drug concentration of 10% (w/w), are dried with a Mini Spray-dryer. The spray-dried Curcumin nanocrystals are directly collected after the process. Yallapu et al., 2010 fabricated curcumin encapsulated PLGA nanoparticles.
Single emulsion method: Single emulsion method is the conventional method for the synthesis of curcumin nanoparticles. In this method, curcumin nanoparticles are prepared by dispersing it in a suitable solvent, followed by high speed homogenization or ultrasonication to form the emulsion. Further the solvent from the emulsion is evaporated by continuous magnetic stirring at room temperature or under reduced pressure. The solidified nanoparticles are ultrasonicated and collected, followed by washing with distilled water to remove additives and lyophilized to get nanoparticles. Curcumin loaded poly (lactic-co-glycolic acid) (PLGA) nanoparticles can also be prepared. Sari et al., 2013 produced curcumin nanoparticles by this method.
Solvent evaporation method: Solvent evaporation method includes two major steps: (i) preparation of drug-polymeric solution (ii) evaporation of dispersing solvent used for dissolving curcumin. It results in the formation of solid mass. The emulsion formed is then converted into nanoparticles suspension by evaporation of the solvent. Advantage of this method is that low temperature required for evaporation of solvent and thermal deposition can be prevented. Disadvantages are: (i) the reagents used in this method are quite expensive (ii) selection of proper solvent is somehow difficult and evaporation of organic solvent is time consuming process. PLGA (Poly (lactic acid-co-glycolic acid) loaded curcumin nanoparticles are synthesized by this technique. Liemann et al., 2013 formulated PHBV nanoparticles by solvent evaporation method.
Microemulsion: Microemulsion is considered as an ideal method for nanoparticles fabrication. The surfactants used in this method are hydrophobic in nature for water soluble drugs and hydrophilic in nature for oil soluble drugs. Microemulsion is formed when a small amount of surfactant is stirred and curcumin is added in it along with oil and water. It results in the formation of turbid solution which generally appears like small droplets. Various types of surfactants are used to increase the surface stabilization of curcumin nanoparticles. This method is easy and can be effectively used for drug delivery with less energy expenditure. Microemulsion technique is affected by certain parameters like temperature and pH variation. Lin et al., 2009 formulated phospholipid-based curcumin-encapsulated microemulsions.
Wet milling method: Curcumin nanoparticles can be synthesized from wet-milling method. Curcumin is suspended in an appropriate dispersing solvent. The obtained solution is further agitated under ultrasonication method. Distilled water is used for the synthesis of curcumin nanoparticles. The obtained solution is then allowed to centrifuge and the formed nanoparticles are collected. Giat et al., 2014 fabricated nanocurcumin by wet milling method.
Thin film hydration method: In this method of synthesis, herbal drug (curcumin) and surfactants are allowed to mix in a suitable organic solvent under sonication condition. Solvent is allowed to evaporate under certain pressure. After that distilled water is added in sonication condition and the obtained nanosuspension is then centrifuged to obtain curcumin nanoparticles. Moorthi et al., 2012 demonstrated curcumin nanoparticles synthesis by this method of synthesis.
Solid dispersion method: In this method, the matrix and hydrophobic drugs like curcumin are mixed. Matrix can be in the amorphous or in crystalline form. This method can be used to dissolve the insoluble hydrophobic drug. This is fast and readily scalable method used for curcumin nanoparticles synthesis. Moorthi et al., 2012 synthesized curcumin nanoparticles by solid dispesion method.
Emulsion polymerization method: Organic and continuous phase are two types of emulsion techniques which can be used for the synthesis of curcumin nanoparticles. By this method, the surfactant is dissolved in pure water by ultrasonication, then curcumin is dissolved in an organic solvent and finally the solution is added to the surfactant. Moorthi et al., 2012 have reported synthesis of curcumin nanoparticles by using this method and piperine was used along with curcumin to increase the biological activity of synthesized curcumin nanoparticles.
Fessi method: In this method of synthesis, curcumin is dissolved in suitable solvent under sonication condition. The solution thus obtained is further added in pure water along with certain surfactant with constant stirring. Curcumin nanoparticles can be spontaneously synthesized by this method. Moorthi et al., 2012 have used this method for fabrication of curcumin nanoparticles. This is easy and simple method of nanoparticles synthesis.
Ionic gelation method: Hydrophobic drug such as curcumin is dissolved in proper solvent which showed complete solubility of curcumin in it and then this solvent is added in polymeric solution under constant stirring condition. This method depends on the cross linking of polymer along with drug such as curcumin. Chabib et al., 2012 reported synthesis of curcumin nanoparticles and used chitosan as a polymer. This polymer improved the solubility and stability of curcumin nanoparticles.
Ultrasonication: This method is generally employed for the drugs which are less water soluble. By this technique, curcumin is first dissolved in an organic solvent and the resulting solution is then added into the polyelectrolye solution under ultrasonication condition for several intervals of time and the formed curcumin nanoparticles are collected. Zhang et al.,
2011 have synthesized curcumin nanoparticles by using this technique of ultrasonication.
Antisolvent precipitation: Antisolvent precipitation is the method of synthesis of the poorly water soluble drug. In this method of synthesis, curcumin is dissolved in an organic solvent followed by the addition of this solution into the deionized water under constant stirring. Hence, curcumin nanoparticles can be synthesized by this method. Yadav et al., 2014 used this method for synthesis of curcumin nanoparticles. Advantage of this method of synthesis is that it is suitable technique for synthesis of poorly soluble curcumin nanoparticles.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
CONCLUSION:
Herbal medicine is the oldest form of health care known to mankind. It is an integral part of the development of modern evaluation. Nanotechnology is an innovative idea that can be used to overcome the problems associated with curcumin solubility, stability and bioavailability. In this review, the various methods of preparation of curcumin nanoparticles and its advantages and drawbacks are discussed. However, most of the known activities of curcumin are based only on in vitro and in vivo studies. Curcumin has yet not been approved for treatment of any human disease. Therefore, more extensive and well-controlled human studies are required to demonstrate this polyphenol's safety and efficacy. Future research should be focused on bringing this fascinating molecule to the forefront of therapeutic agents for the treatment of human diseases.
REFERENCE:
1. Anand P, Kunnumakkara A.B, Newman R.A, Aggarwal B.B. (2007); Bioavailability of curcumin: problems and promises. Mol Pharm. 4(6) ; 807–18.
2. Anand P, Thomas S.G, Kunnumakkara A.B, Sundaram C, Harikumar K.B, Sung B, Tharakan S.T, Misra K, Priyadarsini I.K, Rajasekharan K.N, Aggarwal B.B. (2008); Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem Pharmacol. 76(11) ; 1590–611.
3. Chabib L, Martien R, Ismail H, (2012), Formulation of nanocurcumin using low viscosity chitosan polymer and its cellular uptake study into T47D cells. Indonesian J. Pharm. 23(1): 27 – 35.
4. Chin S.F, Akmar Mohd Yazid S.N, and Pang S.C. (2014), Preparation and Characterization of Starch Nanoparticles for Controlled Release of Curcumin., Int. J. of Polymer Science ; 8.
5. Chirio D, Gallarate M, Peira E, Battaglia L, Serpe L, Trotta M; (2011), Formulation of curcumin-loaded solid lipid nanoparticles produced by fatty acids coacervation technique. J. Microencapsul. 28(6), 537-48.
6. Giat L, Sinh D.T, Toan T.P, (2014) ; High concentration Nanacurcumin fabrication by wet milling method curcumin with glassball, International Journal Of Scientific & Technology research, 3(8) ; 345-348
7. Leimann F.V, Cardozo L, Sayer C, & Araújo P.H, (2013) ; Poly(3-hydroxybutyrate-co-3- hydroxyvalerate) nanoparticles prepared by a miniemulsion/solvent evaporation technique. Effect of PHBV molar mass and concentration, Brazilian Journal of Chemical Engineering, 30(2), 369-377.
8. Li L, Braiteh F.S, Kurzrock R. (2005); Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 104(6) ; 1322–31.
9. Lin C.C (2009) ; Stability and characterization of phospholipid-based curcumin-encapsulated microemulsions, Food Chem, 116(4) ; 923–928.
10. Liu A, Lou H, Zhao L, Fan P. (2006); Validated LC/MS/MS assay for curcumin and tetrahydrocurcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J Pharm Biomed Anal. 40(3) ; 720–7.
11. Masuda T, Isobe J, Jitoe A and Nakatani N. (1992)., Antioxidative curcuminoids from rhizomes of Curcuma xanthorrhiza. Phytochemistry., 31(10) ; 3645-7.
12. Moorthi C, Kiran Krishnan, Manavalan R, (2012); Preparation and characterization of curcumin-piperine dual drug loaded nanoparticles, Asian Pacific Journal of Tropical Biomedicine, 2(11): 841-848.
13. Ohori H, Yamakoshi H, Tomizawa M, (2006); Synthesis and biological analysis of new curcumin analogues bearing an enhanced potential for the medicinal treatment of cancer. Mol Cancer Ther. 5(10) ; 2563–71.
14. Prasad S, Gupta S.C, Tyagi A.K, Aggarwal B.B. (2014) ; Curcumin, a component of golden spice: from bedside to bench and back. Biotechnol Adv. 32(6) ; 1053–64.
15. Preetha A, Banerjee R, Huilgol N, (2007); Tensiometric profiles and their modulation by cholesterol: implications in cervical cancer. Cancer Invest. 25(3) ; 172–81.
16. Sari T.P, Mann B, Sharma R, Kumar R, Vikrant N, (2013); Process Optimization for the Production of Nanoencapsulated Curcumin and Analysis for Physicochemical Characteristics and Antioxidant Mechanism, Int. J. of Biotechnology and Bioengineering Research, 4(6), 581-586.
17. Sreejayan S, and Roa M.N, (1994) ; Curcuminoids as potent inhibitor of lipid peroxidation. J.Pharm. Pharmacol, 46(12) ; 1013-6.
18. Suresh D, Srinivasan K, (2007); Studies on the in vitro absorption of spice principles-curcumin, capsaicin and piperine in rat intestines. Food Chem Toxicol. 45(8) ; 1437–42.
19. Tiyaboonchai W, Tungpradit W, Plianbangchang P, (2007) ; Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int J Pharm. 337(1-2) ; 299–306.
20. Unnikrishnan M.K. and Roa M.N, (1995) ; Inhibition of nitrite induce oxidation of hemoglobin by curcuminoids. Pharmazie., 50(7) ; 490-2.
21. Yadav D, Kumar N, (2014), Nanonization of curcumin by antisolvent precipitation: Process development, characterization, freeze drying and stability performance. Int J Pharm, 477(1-2): 564-77.
22. Yallapu MM, Gupta BK, Jaggi M, and Chauhan SC, (2010), Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci., 351(1), 19-21.
23. Zhang H, Zhang L, Yuan P, Wang C, (2011); Preparation and in vitro release characteristics of curcumin in nanosuspensions, Zhongguo Zhong Yao Za Zhi , Chinese, 36(2) ; 132-135.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









