ABOUT AUTHORS
Brijesh Dasvani*, Avani Khristi
Department of Quality Assurance
Parul Institute of Pharmacy, Waghodiya,Limda, Vadodara, Gujarat.
ABSTRACT
Oral dosage forms are the best medicine administration way of taking medication, despite having some disadvantages compared with other methods like risk of slow absorption of the medicament, which can be overcome by administering the drug in liquid form, therefore, possibly allowing the use of a lower dosage. However, instability of many drugs in liquid dosage form limits its use. Effervescent technique can be used as alternate to develop a dosage form which can accelerate drug disintegration and dissolution, is usually applied in quick release preparations. Along with the development of new pharmaceutical technique, effervescent tablet are more and more extensively to adjust the behaviour of drug release, such as in sustained and controlled release preparations, pulsatile drug delivery systems, and so on.
Reference Id: PHARMATUTOR-ART-2683
INTRODUCTION
Oral drug delivery has been known for decades as the most widely utilized route of administered among all the routes that have been employed for the systemic delivery of drug via various pharmaceutical products of different dosage forms. The reasons that the oral route achieved such popularity may be in part attributed to its ease of administration. (AS, 2012) (Abolfazl A, 2013) Oral sustained drug delivery system is complicated by limited gastric residence times (GRTs). Rapid GI transit can prevent complete drug release in the absorption zone and reduce the efficacy of the administered dose. (Aboud HM, 2012) (Agyilirah GA, 1991)
Effervescent tablets are becoming increasingly popular in a variety of sectors including supplements and pharmaceutical use due to the ease in which they can be consumed. Effervescent tablets are designed to break in contact with liquid such as water or juice, often causing the tablet to dissolve into a solution. (Ahmed I, 2007)
These delivery systems utilize matrices prepared with swellable polymers such as Methocel or poly saccharides, e.g., chitosan, and effervescent components, e.g., sodium bicarbonate and citric or tartaric acid[6] or matrices containing chambers of liquid that gasify at body temperature (Ashish P, 2016) (Biswas D and Halquist M, 2016) Flotation of a drug delivery system in the stomach can be achieved by incorporating a floating. (Biswas D and Halquist M, 2016) (SK., 2015) (Deepali DW, 20111) (Dhakar RC, 2010) (Dhakar RC M. S., 2010) (Dixit N, 2013) (ED, 2014) (Gharti KP, 2009) (Harald S, 2003) (Hassali MA, 2016) (Howard CA, 2000)
Benefits of effervescent tablets over simple tablets: (Indian Pharmacopoeia, 1996) (RR, 2014) (Lachman L, 1986) (Larry LA and Stephan WH, 1993) (Maurya SD, 2013) (Maurya SD T. V., 2011) (Mohrle, 2005) (Obara T, 2015) (JS, 2000) (JS, 2015) (A, 2015)
Easy Alternative to Regular Tablets:
They can be a great alternative for those who may have trouble swallowing either due to illness or age. Older individuals may have difficulty swallowing but need to take medication or supplements on a regular basis and in this respect, effervescent tablets can be a lot easier than having to swallow a tablet. In addition to this, they can be a great way of ingesting medicine for individuals with sore throats or medical issues that make swallowing difficult and so are a viable alternative to regular tablets.
Increased Liquid Intake:
Effervescent tablets provide the nutritional benefits intended, but in addition to this they also increase liquid intake. This can be especially beneficial if you are dehydrated or ill and not ingesting as much fluid as usual. Effervescent tablets can be a fantastic way of rehydrating as well as reaping the benefits you are taking the tablets for whether this is a dietary supplement, herbally or medicinally.
Distributed More Evenly:
Conventional tablets dissolve gradually in the stomach once ingested and can sometimes only partially dissolve which can lead to irritation in some cases. The benefit of effervescent tablets is that they dissolve completely and evenly meaning that localised concentrations of the ingredients cannot occur. This means not only a better taste but also less chance of irritation and a more efficient means of ingesting the ingredients.
Chemical responsible for effervescents (Ashish P, 2016), (Swarbrick J, 2002), (Lachman L, 1986), (Yanze FM D. C., 2000), (Thoke SB, 2013)
Potassium bicarbonate, Tartaric acid, Malic acid, Sodium bicarbonate, Sodium carbonate, Potassium carbonate, Citric acid, Fumaric acid.
Advantages of effervescent tablets
No need to swallow tablet, Good stomach and intestinal tolerance, More portability, Improved Therapeutic Effect, Fast onset of action, Improved palatability, Superior stability, More consistent response, Incorporation of large amounts of active ingredients, Accurate Dosing. (Lachman L, 1986), (RR, 2014), (Indian Pharmacopoeia, 1996)
Disadvantages of effervescent tablets
Clear solution is preferred for administration, although a fine dispersion is now universally acceptable, Unpleasant taste of some active ingredients, Relatively expensive to produce due to large amount of more or less expensive excipients and special production facilities. (Indian Pharmacopoeia, 1996), (Lachman L, 1986)
Quality control test of effervescent tablet (Yanze FM D. C., 2000), (Vergeire DG, 2016), (Formulary, 2008), (Thoke SB, 2013), (Swarbrick J, 2002), (Skalkz BN, 2016), (Singh LP, 2011), (Singh BN, 2000), (Shimodaira S, 2016), (Sastry SV, 2000), (Sandhyarani G, 2017), (Indian Pharmacopoeia, 1996)
Weight variation: Weight variation was determined to know whether different batches of tablets have uniformity. Weighed 20 tablets individually, calculated the average weight and compared the individual tablet weights to the average. The tablets meet the test if not more than two tablets are outside the % limit and none of the tablet differ by more than two times the % limit. Weight variation specification as per I.P.
Table: 1 : Weight variation specification
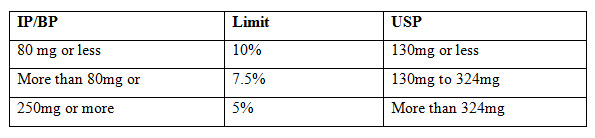
Tablet Thickness and Diameter:
Thickness and diameter of tablets were important for uniformity of tablet size. Thickness and diameter were measured using Vernier Calipers.
Tablet Hardness:
The resistance of tablets to shipping or breakage under conditions of storage, transportation and handling before usage depends on its hardness. The hardness of tablet of each formulation was measured by Monsanto Hardness Tester. The hardness was measured in items of kg/cm2. Hardness or tablet crushing strength is the force required to break a tablet in a diametric compression. The force is measured in kg and the hardness of about 3-5 kg/cm2 is considered to be satisfactory for uncoated tablets.
Friability (F):
Friability of the tablet determined using Roche friabilator. This device subjects the tablet to the combined effect of abrasion and shock in a plastic chamber revolving at 25 rpm and dropping a tablet at a height of 6 inches in each revolution. Pre weighted sample of tablets was placed in the friabilator and were subjected to the 100 revolutions. Tablets were dusted using a soft muslin cloth and reweighed. USP limit is 0.5 to 1%. The friability (F) is given by the formula.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Measurement of effervescence time:
A single tablet is placed in a beaker containing 200 ml of purified water at 20 °C ± 1 °C. Whenever a clear solution without particles is obtained effervescence time has finished. The mean of three measurements of each formulation is to be reported.
Determination of effervescent solution pH:
pH of solution is determined with one tablet in 200 ml of purified water at 20 ± 1 °C by using pH meter, immediately after completing the dissolution time. Repeat experiment 3times for each formulation.
Measurement of CO2 content:
One effervescent tablet solved in 100 ml of 1N sulphuric acid solution and weight changes were determined after dissolution end. The obtained weight difference is shown the amount (mg) of CO2 per tablet. Reports the averages of 3 determinations.
Evaluation of the water content:
10 tablets of each formulation are dried in a desiccators containing of activated silica gel for 4 hours. Water content of 0.5% or less is acceptable.
Uniformity of Content:
10 tablets were selected randomly. Each tablet was transferred into a 50mL volumetric flask, dissolved and diluted to 50 mL with phosphate buffer pH 6.8. One ml of this solution was diluted to 100 ml with phosphate buffer pH 6.8. The amount of drug present in each tablet was determined by UV spectroscopy at 246 nm. Standard limit for uniformity of content is
IP: - Active less than 10mg or 10%,
BP:- Active less than 2 mg or 2%,
USP:- Active less than 25mg or 25%.
10 tabs limit NMT 1 tab deviate 85 – 115% & none outside 75 – 125% of the Average value/IP/BP/USP (Relative Standard Deviation less than or equal to 6%),
If 2 or 3 individual values are outside the limits 85 – 115% of the Average value, & none outside 75 – 125% repeat for 20 tablets.
Determination of the equilibrium moisture content:
Three desiccators are prepared containing saturated salt solutions of potassium nitrate (for creation 90% RH, at 18 °C), sodium chloride (for creation 71% RH, at 18 °C) and sodium nitrite (for creation 60% RH, at 18 °C). Three tablets of each formulation are placed in desiccators. Then, the equilibrium moisture content is determined by Karl Fischer method and the autotitrator device in the first day and after 7day.
In-vitro disintegration time:
The process of breakdown of a tablet into smaller particles is called as disintegration. The in-vitro disintegration time of a tablet was determined using disintegration test apparatus as per I.P. specifications.
I.P. Specifications: Place one tablet in each of the 6 tubes of the basket. Add a disc to each tube and run the apparatus using phosphate buffer (pH-6.8) maintained at37°±2°C as the immersion liquid. The assembly should be raised and lowered between 30 cycles per minute in the phosphate buffer (pH-6.8) maintained at 37°±2°C. The time in seconds taken for complete disintegration of the tablet with no palpable mass remaining in the apparatus was measured and recorded. Standard limit for disintegration time is within 3 min in water at 250C ± 10C (IP) and 15 – 250C (BP).
Dissolution Studies:
The release rate of Atorvastatin from mouth dissolving tablets was determined using USP Dissolution Testing Apparatus II (Paddle type). The dissolution medium used was 900 ml of phosphate buffer pH 6.8 which was maintained at 37±0.50C. The paddle speed was kept at 50 rpm throughout the study. Five ml of samples was withdrawn at every 5 minutes interval and diluted to 10ml then 5ml of fresh dissolution media maintained at the same temperature was replaniced. The samples were analysed spectrophotometrically at 246nm using phosphate buffer pH 6.8 as blank. The raw dissolution data was analyzed for calculating the amount of drug released and percentage cumulative drug released at different time intervals.
REFERENCES
1. Abdul AS (2012); Formulation, Evaluation and Mathematical Modeling of Clopidogrel Bisulphate & Aspirin Immediate Release Bilayer Tablets; Pharmaceutca Anal Acta; 3(9); 194.
2. Abolfazl A, (2013); Characterization and Physicochemical Evaluation of Ranitidine Effervescent Tablets; Advanced Pharmaceutical Bulletin, 3(2); 315-322.
3. Aboud HM, Elbary A, Ali AA, (2012); Enhanced dissolution of meloxicam from orodispersible tablets prepared by different methods, Bulletin of Faculty of Pharmacy, Cairo University; 50(2); 89–97.
4. Agyilirah GA, Green M, DuCret R, Banker GS, (1991); Evaluation of the gastric retention properties of a cross-linked polymer coated tablet versus those of a non-disintegrating tablet; International Journal of Pharmaceutics; 75(2-3); 241–47.
5. Ahmed I, Aboul-Einien M, (2007); In vitro and in vivo evaluation of a fast disintegrating lyophilized dry emulsion tablet containing griseofulvin; European Journal of Pharmaceutical Sciences; 32(1); 58-68
6. Aly AM, Amro BI, Hajji FD, (2011); Preparation and Evaluation of Rapidly Disintegrating Glimepiride Tablets; International Journal of Pharmaceutical Sciences and Nanotechnology; 3(4);1220-1229.
7. Ashish P, Harsoliya MS, Pathan JK, Shruti S, (2016); A Review- Bhattacharjee J. Mass Drugs Administration in India - A Failure Story; Epidemiology Sunnyvale; 6; 252.
8. Biswas D and Halquist M, (2016); Using Biorelevant in Vitro Models Testing to Characterize Release of Non Oral Dosage Forms as another Tool for Safety; Journal of Pharmacovigilance; 4(2); 153-160.
9. Cho SK. (2015); The Synergistic Effects of Pioglitazone on the Glucose-Lowering Action of Metformin in Relation to OCT1 and Gluts m-RNA Expression in Healthy Volunteer; Clinical Pharmacology and Biopharmaceutics; 3;129.
10. Deepali DW, Madhav MS, Jain DS, (2011), Gastroretentive Floating Microspheres: A Review, International Journal Of Pharmacy &Technology, 3(4):1783-1799.
11. Dhakar RC, Maurya SD, Aggarawal S, Kumar G, Tilak VK, (2010); Design and evaluation of SRM microspheres of Metformin hydrochloride; Pharmacie Globale(IJCP), 1(07); 1-6.
12. Dhakar RC, Maurya SD, Dangi G, Kumar G , Gupta M, Kiroriwal S, (2010); Buccal Adhesive Dosage Forms As A NISDD: A Pharmaceutical Review; Research Pharmaceutica; 1(1); 46-59.
13. Dhakar RC, Maurya SD, Gupta AK, Siddiqui AW, (2010); Interpenetrating polymeric network hydrogel for stomach-specific drug delivery of clarithromycin: Preparation and evaluation; Asian Journal of Pharmaceutics; 4(4); 184-189.
14. Dhakar RC, Maurya SD, Sagar BPS, Prajapati SK, Jain CP, (2010); Variables influencing the drug entrapment efficiency of microspheres: a pharmaceutical review; Der Pharmacia Lettre; 2(5);102-116.
15. Dixit N, Maurya SD, Sagar BPS, (2013); Sustained release drug delivery system; Indian Journal of Research in Pharmacy and Biotechnology; 1(3); 305-310.
16. Ehrenpreis ED, (2014); A Survey of Lawsuits Filed for the Complaint of Tardive Dyskinesia Following Treatment with Foldvari M, Nanopharmaceutics Innovations in Gene Therapy: Moving Towards Non-Viral and Non-Invasive Delivery Methods; J Nanomedine Biotherapeutic Discovery; 4;135.
17. Gharti KP, Thapa P, Budhathoki U, Bhargava A, (2009); Formulation and in vitro evaluation of floating tablets of hydroxypropyl methylcellulose and polyethylene oxide using ranitidine hydrochloride as a model drug, Journal of Young Pharmacists; 4(4); 201-208.
18. Harald S, (2003); Effervescent Dosage; Pharmaceutical Technology Europe; 15(4); 25–28.
19. Hassali MA, (2016); Role of Pharmacists in Health Based Non-Governmental Organizations NGO: Prospects and Future Directions, Pharm Anal Acta; 7(2);467.
20. Howard CA, Lloyd A, Nocholas and Popovich, (2000); “Effervescent granules”8th edition “Pharmaceutical Dosage From and Drug Delivery” International Student Edition.- 172-178.
21. Indian Pharmacopoeia, (1996); Government of India Ministry of Health and Family Welfare;Delhi: Controller of Publications 2: 35; 448, 554.
22. Kokardekar RR, (2014); Development and Evaluation of Sustained Release Microspheres of Glibenclamide by Emulsion Solvent Evaporation Method; Clinical Pharmacology and Biopharmaceutics; 3; 127.
23. Lachman L, Liberman HA, Kanig JL, (1986); The theory and practice of industrial pharmacy. 3rd ed. Philadelphia: lea and febiger.
24. Larry LA and Stephan WH, (1993); Pharmaceutical Dosage Form: Tablets 3rd edition Vol. 1:. Nanoimaging for Molecular Pharmaceutics; 465.
25. Maurya SD, Rawal RK, Jha S, Chauhan PS, Kumar A, (2013); Drug Loaded Beads: Current; American Journal of Pharm Tech Research, 3 (1); 331-337.
26. Marcel Dekker; Teoh BC, et al. (2015); Perceptions of Doctors and Pharmacists towards Medication Error Reporting and Prevention in Kedah, Malaysia: A Rasch Model Analysis; AdvPharmacoepidemiol Drug Saf; 4(5); 192
27. Maurya SD, Tilak VK, Dhakar RC, Verma KK, Soni U, Gupta, (2011); Preparation and evaluation of floating tablet of famotidine through solid dispersion; International Journal of Current Research and Review; 2(1); 21-30.
28. Mohrle, R., Liberman, L,Schwartz L, (2005); Pharmaceutical Dosage Form; Marcel Decker Inc; New York; 1; 285- 292.
29. Obara T, Prevalence, (2015); Determinants, and Reasons for the Non-Reporting of Adverse Drug Reactions by Pharmacists in the Miyagi and Hokkaido Regions of Japan; Advance Pharmacoepidemiology and Drug Safety; 4(5);191.
30. Patil JS, (2000); Hydrogel System An Approach for Drug Delivery Modulation; Advance Pharmacoepidemiology and Drug Safety, 4(5);135.
31. Patil JS, (2015); Novel Tubercular Therapeutic Agents: Need of the Day; Advance Pharmacoepidemiology and Drug Safety; 4(5); 137
32. Sallam A, (2015); Bioequivalence of Two Oral Formulations of Modafinil Tablets in Healthy Male Subjects under Fed and Fasting Conditions; Journal of Bioequivalence Availability; 7(2);63-67.
33. Sandhyarani G, Kumar KP, (2017); Formulation and evaluation of fast dissolving Tablet of imidapril, Indian Journal of Pharmaceutical Science& Research; 4(3); 147-150.
34. Sastry SV, Nyshdham JR, Fix JA, (2000); Recent technological advances in oral drug delivery: A review; Pharmaceutical Science and Technology Today, 1(3); 38-45.
35. Shimodaira S, (2016); Quality Verification of Dendritic Cell-Based Cancer Vaccine; Pharm Anal Acta; 7(2); 467.
36. Simona B, Tanja R, (2010); Using different experimental designs in drug excipient Compatibility Studies during the Pre-formulation development of a stable solid dosage formulation; Acta Chimica Slovenica; 57(4); 895-903.
37. Singh BN, Kim KH, (2000); Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention; Journal of Controlled release; 63(3);235–59.
38. Singh LP, Rajesh KS, Umalkar DG, Chauhan VK, Rana VK, Vasava KS, (2011); Floating Effervescent Tablet : A Review; Journal of pharmaceutical and biomedical sciences; 5(11); 1-6.
39. Skalkz BN, Srinath KR, (2016); Note on the “Molecular Pharmaceutics and “Formulation and Evaluation of Effervescent tablets of Paracetamol”, International Journal of Swain S and Beg S. Emergence in the Lipid-Based Nanostructured Systems for Optimizing Oral Delivery of Drugs; Pharmaceutical Regulatory Affairs; 5;157-163.
40. Swarbrick J, Boylan JC. (2002); Encyclopaedia of pharmaceutical technology; New York.
41. Thoke SB, Sharma Y., Rawat S, Nangude, S (2013); Formulation development & evaluation of effervescent tablet of Alendronate sodium with vitamin D3; Journal of Drug Delivery & Therapeutics;; 3(5); 65-74.
42. United States Pharmacopeia 31/National Formulary 26. (2008); Rockville MD USA: United States Pharmacopeial Convention.
43. Vergeire DG, (2016); Usefulness of Cost Effectiveness: Evidence versus Applicability; Pharm Anal Acta, 7(1); 456.
44. Yanze FM, Duru C, Jacob M, (2000); A process to produce effervescent tablets: Fluidized bed dryer melt granulation; Drug Development & Industrial Pharmacy, 26(11);1167-76.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









