Question Paper of Pharmacetical Chemistry for Jharkhand Public Service Commission (JPSC) - 2013
1. 17 b-hydroxyl androst-for-ene-one is an IUPAC name of
(A) Progesterone
(B) Testoterone
(C) Cortione
(D) estrone
2.. Identify the ring given below :
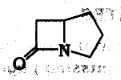
(A) Cepham
(B) Penam
(C) carbapenam
(D) carbapenom
3.In the glucuronide conjugation reaction of meprobamate, the functional group responsible is
(A) hydroxy
(B) carboxyl
(C) amide
(4) thiol
4. Trimethoprim coltanis
(A) pyrimidine nucleus and 2,4,6-trimethoxy benzyl substitution
(B) pyridine nucleus and 2,4,6-trimethoxy benzyl substitution
(C) pyrimidine nucleus and 3,4,5-trimethoxy benzyl substitution
(D) pyridine nucleus and 3,4,5-trimethoxy benzyl substitution
5. Mefenarnic acid is aderivative of
(A) aryl propionic acid
(B) aryl acetic acid
(C) anthramilic acid
(D) none of the above
6. Saccharimetry is the practical applicatin of
(A) potontiometry
(B) polarmetry
(C) aquametry
(D) acidimetry
7. In which ibf the following titrations, i > oxidation-reduction (redox) step can be expected?
(A) Aquametry
(B) Ceriometry
(C) Gay-Lussac method
(D) Kjeldahl titration
8. Assay of calcium gluconate is performed by
(A) direct titration
(B) indirect th^ation
(C) alkalimetric titration
(D) replacement titration
9. Oxazepam is a metabolite of
(A) diazepam
(B) temazepam
(C) Both (A) and (B)
(D) None of the above
10. Antibiotic streptomycin acts by
(A) inhibition of protein synthesis by binding with 30S ribbsohial unit
(B) inhibition of protein synthesis bybindiiig with 50S ribosomai unit
(C) inhibition of cell wall synthesis
(D) inhibition of DNA gyrase
Go to "Next Page" for more questions...
Subscribe to Pharmatutor Job Alerts by Email
11. Which one of the following is a selective COX-2 inhibitor?
(A) Piroxicam
(B) Valdecoxib
(C) Aspirin
(D) Thioridazine
12. Because of bitter taste, Chloramphenicol is available in which of the following salts for pediatric usage?
(A) Phosphate
(B) Palmitate
(C) Hydrochloride
(D) Fumarate
13. Which of the following is an example of prodrug?
(A) Phenothiazine
(B) Ibuprofen
(C) Enalapril
(D) INH
14. Which of the following is anti-metabolite drug having anti-fungal activity?
(A) Clotrimazole ?
(B) Flucytosine
(C) GnSeofulvin
(D) Terbinafine
15. Stavudine is an example of
(A) non-nucleoside pyrimidine analogue
(B) nucleoside pyrimidine analogue
(C) nucleoside pyridine analogue .
(D) nucleoside deoxypurine analogue
16. Amodiaquine structure is a derivative of
(A) 4-aminoquinoline with N-phenyl substitution
(B) 3-aminoquinoline with N-phenyl substitution
(C) 4-aminoquinoline with pyridazine substitution
(D) 3-aminoquiline with pyridazine substitution
17. Higher the dielectric constant
(A) lower will be ,the dissociation of solute
(B) higher will be the dissociation of solute
(C) ion pairing occurs
(D) Both (A) and (C)
18. Oxygen flask combustion method was developed by .
(A) K. Fajan
(B) Kjeldahl
(C) Schoriger
(D) None of the above
19. Which of the following drugs belongs to nitrosourea class?
(A) Streptozocin
(B) Dacarbazine
(C) EstraittUStine
(D) Nitazoxanide
20. Which of the following is non-steroidal drug having potent oestrogenic activity?
(A) cis-Diethylstilbestrol
(B) tmns-Diethylstilbestrol
(C) Triamcinolone
(D) Auranofin
21. The of metabolishm of diazepam occurs by
(A) N-dealkylation
(B) hydroxylataon
(C) Both (A) and (B)
(D) None of the above
22. Which of flic foUowing is in antifungal antibiotip?
(A) Erythromycin
(B) Rifampin
(C) Cycloserine
(D) Natamycin
23. The active metabolite of anti- cancer cyclophosphamide is
(A) 4-hydroxy cyclophosphamide
(B) IV-acetyl cyclophosphamide
(C) N-hydroxy cyclophosphamide
(D) N-hydroxymethyl cyclophosphamide
24. Absolute stereochemistry is represented by
(A) D and L
(B) d and L
(C) R and S
(D) All of the above
25. Sulphonamide not having anti¬diabetic activity is
(A) tolbutamide
(B) acetohexamide
(C) mafenide
(D) chlorpropamide
Go to "Next Page" for more questions...
Subscribe to Pharmatutor Job Alerts by Email
26. Duration of action of insulin may be prolonged by
(A) esterification of amino acid residue
(B) forming complex of insulin with protein
(C) binding with acidic resins
(D) None of the above
27. How many chiral centres are present in steroid nucleus?
(A) 6
(B) 4
(C) 5
(D) 7
28. A prodrug used in the treatment of parkinsonism is
(A) levodopa
(B) carbidopa
(C) selegiline
(D) bromocriptine
29. The vitamin administered with isoniazid to minimize its adverse reaction is
(A) pyridoxine
(B) niacin
(C) thiamine
(D) biotin
30. Antihypertensive agent terazosin contains
(A) quinazoline and pyran ring
(B) quinazoline arid piperazine ring
(C) quinoline and piperazine ring
(D) quinoline and triazole ring '
31. Clavulanic acid has a beta lactum ring fused to
(A) thiadiazole system
(B) thiazolidine system
(C) oxazolidine system
(D) thiazole system
32. Which of the following steroids shows predominant mineralo-
corticoidal action?
(A) Dexamethasone
(B) Prednisolone
(C) Triamcinolone
(D) Fludrocortisone
33. Benzodiazepines potentiate the
(A) binding of proteins to nervous tissue
(B) binding of GABA to receptors
(C) binding of GABA to carbohydrates
(D) binding of GABA to lipids
34. Which antineoplastic agent is metabolized by xanthine
oxidase?
(A) 6-Thioguanine
(B) Vincristine
(C) 6-Mercaptopurine
(D) Doxorubicin
35. An anticholine inhibitor useful in Alzheimer’s disease is
(A) isoproterenol
(B) clioquinol
(C) donepezil
(D) arecoline
36. Correct unit of wave number is
(A) cm
(B) m-1
(C) cm-1
(D) cm-2
37. Radiation which have enougfh energy to cause transitions of the outermost electrons are
(A) UV-visible
(B) X-ray
(C) IR
(D) NMR
38. Which of the following is the bulk property detector?
(A) Diode array detector
(B) UV detector
(C) Fluorescence detector
(D) Refractive index detector
39. The efficiency of chromatographic column is measured by
(A) its length
(B) HETP
(C) number of theoretical plates
(D) None of the above
40. Diatomic molecule absorbed r in IR region is
(A) H2
(B) O2
(C) N2
(D) Hcl
Go to "Next Page" for more questions...
Subscribe to Pharmatutor Job Alerts by Email
41. Choose the correct statement ifor monobasic acids and mono-acidic bases.
(A) Molar and normal solutions are identical in Concen¬tration
(B) Molar and normal solutions are not identical in concentration
(C) Concentration of molar solution is double of normal solution
(D) Concentration of normal solution is double of molar solution
42. In the treatment of angina, verapamil acts as
(A) Bita-blocker
(B) calcium channel blocker
(C) vasodilator
(D) platelets aggregation inhibitor
43. Naloxone is
(A) pure opioid antagonist and is N-allyl derivative of dihydromorphinone
(B) pure opioid antagonist and is N-allylnormorphine
(C) partial, ppioid, antagonist and is N-allylnormorphine
(D) less active than nalorphine as morphine antagonist
44. Drug not having phienylethyi-amine moiety is
(A) amphetamine pheniramipe
(B) glyburide
(C) mescaline
45. Which structural feature is common in atenolol, propranolol and sotalol?
(A) 1-Methylethyl amino
(B) 2-Hydroxyethyl amino
(C) 1-Propyl amino
(D) None of the above
46. Which of the following is an example of polypeptide
antibiotic?
(A) Neomycin
(B) Bacitracin
(C) Amikacin
(D) Clindamycin
47. Antacid bets
(A) by decreasing the volume of Hcl in stomach
(B) by neutralizing the gastric HC1 contents
(C) through H/K ATPase pump
(D) All of the above
48. The Sodium Rose Bengal (1-131) is Used in
(A) study of potassium ion exchange
(B) plasma volume determination
(C) brain scanning
(D) liver function determination
49. Which one of the following is used as systemic alkalizer?
(A) Sodium chloride
(B) Sodium bicarbonate
(C) Sodium sulphate
(D) Sodium acetate
50. Which of the following is inorganic saline expectorant?
(A) Ammonium chloride
(B) Potassium iodide
(C) Potassium guaiacol sulfonate
(D) All of the above
51. uric acid stone formation can be reduced by
(A) acidification of Urine
(B) alkalinization of urine
(C) acidification of serum
(D) alkalinization of serum
52. Consumption; of jraw egg cause deficiency of
(A) riboflavin
(B) pyridoxine
(C) biotin
(D) cyanocobalamin
53. This makes a compound to act as a central sympathomimetic agent
(A) Introduction of beta hydroxyl group in phenylethylamine
(B) Branching with lower alkyl group on alfa carbon of phenylethylamine
(C) Halogenations of aromatic ring of phenylethylamine
(D) Methoxy substitution of aromatic ring of phenyl¬ethylamine
54. Which one of the following is non-depolarizing, neuromuscular blocking agent?
(A) Succinylcholine chloride
(B) d-Tubocurarine
(C) Decamethonium
(D) Scopolamine hydrobromide
55.Which one of the following diuretics is devoid of adverse effects related to sulphamoyl moiety?
(A) Spironolactone
(B) Azosemide
(C) Acetazolamide
(D) Hydrochlorothiazide
Go to "Next Page" for more questions...
Subscribe to Pharmatutor Job Alerts by Email
56. The energy is inversely proportional, to
(A) frequency
(B) wavelength
(C) Both (A) and |B)
(D) None of the above
57. Which one of the following parts is used to isolate particular wavelength or range of wavelengths?
(A) Radiation source
(B) Detector
(C) Monochrotnator
(D) All of the above
58. Chemical shift is denoted by
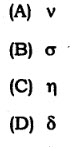
59. Which one efco the fotlo*i6g standard. w^ences, fir rased in NMR universally?
(A) Benzene
(B) Chloroform
(C) TMS
(D) None of the above
60. The mass analyzer separates the sample ions based on their...
(A) mass td chargp ratitfc
(B) mass to temperature ratio
(C) mass to velocityratio
(D) mass to ioris ratio
61. MALDI is
(A) matrix4assisted lower desorption ionization
(B) matrix-assisted laser desorption Ionization
(C) mass-assisted laser desorption ionization
(D) mass-assisted laser design ionization
62. In adsorption chromatography, the stationary Phase is
(A) liquid
(B) gas
(C) solid Q;
(D) All of the above
63. in van Deemeter equation
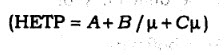
the term A is
(A) molecular diffusion '
(B) eddy diffusion
(C) mass transfer
(D) None of the above
64. Which of the following is not acting as antimetabolite?
(A) 6-Thioguanine
(B) 6-Mercajptopurine
(C) Clofibrate
(D) Metbptrexate
65. Chloropromazine structure contains Which of the following
(A) Piperazine
(B) Xantbipe
(C) Phenothiazine
66. Identify the structurer given below :
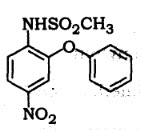
(A) Sulindac
(B) Nimesulide
(C) Naproxen
(D) Benoxinate
67. Starting material for the synthesis of dacarbazine is
(A) 5-aminoimidazole-4- carboxamide
(B) 1-(2,4-dichlorobenzoyl-methyl)-imidazole
(C) 5-hydroxy-3methyl-imidazole
(D) 4-hydroxy-2-carboxyl-imidazole
68. Clofaziminebelongs to class of
(A) iminophenazines
(B) phenothiazines
(C) pyrimidines
(D) benzotiazole
69. Ethyl deriwative of lnraethyte4- phenyl piperidine-4-carboxylic acid is ....
(A) anthelmintic agent
(B) narcotic analgesic
(C) nonsteroidal antiinflamma¬tory agent
(D) antifungal agent
70. Captopril is the derivative of amino acid
(A) histidine
(B) tyrosine
(C) phenylalanine
(D) proline
Go to "Next Page" for more questions...
Subscribe to Pharmatutor Job Alerts by Email
71. 2,6-Dimethylaniline and chloro- acetyl chloride are die starting materials for the synthesis of drug
(A) lidocaine
(B) benzocaine
(C) etidocaine
mepivScairi6
72. Resolution is a method to separate
(A) diasteromeps,
(B) enantiomers
(C) steroidal mixtures
(D) None of the above
73.Alfentanil is a/an..
(A) synthetic opioid analgesic agent
(B) naturally occurring analgesic agent
(C) COX-2 inhibitor
(D) anilide derivative used as NSAID
74. Which one of the following does not contain piperidine moiety in its structure?
(A) Mepivacaine
(B) Ropivacaine
(C) Lidocaine
(D) Bupivacaine
75. The phenothiazine derivative used for Parkinson’s disease is
(A) prochlorperazine
(B) prometharine
(C) ethopropazine
(D) chlorpromazine
76.Which one of the following belongs to imidazolidine 2,4-dione class?
(A) Phenytoin
(B) Trimethadione
(C) Phensuximide
(D) Paramethadione
77. Replacement of oxygen at C-2 of barbituric acid by a sulfur
(A) increases lipid solubility
(B) decreases lipid solubility
(C) shows no change in lipid solubility
(D) None of the above
78. In the limit test for sulphate, which one of the following is used to prevent supersaturation?
(A) Barium sulphate
(B) Potassium sulphate
(C) Alcohol
(D) None of the above
79. Which of the following is an oxidizing agent?
(A) Hydrogen peroxide
(B) Potassium permanganate
(C) Concentrated nitric acid
(D) All of the above
80. Molecule tie become IR active in IR spectroscopy
(AJ must show dipole moment
(B) must show change in dipole moment
(C) must show magnetic moment
(D) must show change in magnetic moment ;
81. Electrdmagnetie radiation used in NMR spectroscopy is
(A) microwave
(B) radiofrequency wave
(C) ultraviolet
(D) infrared radiation
82. Which one of the following indicators is used in complexometric titration?
(A) Methyl orange
(B) Murexide j
(C) Eosin j
(D) Crystal violet
83. Sulphiir containing B-complex vitamin is ^
(A) biotin ?
(B) riboflavin
(C) niacin ; nr
(D) pyridoxine
84. Triamcinolone is

85. 2,6-Dimethoxyphenyl penicillin iS the lUPAC name of
(A) ampicillin
(B) methicillin
(C) amoxicillin
(D) carbenicillin
Go to "Next Page" for more questions...
Subscribe to Pharmatutor Job Alerts by Email
86. In gel permeation chromato¬graphy, molecules are separated on the basis of their
(A) size and shape
(B) chemical nature
(C) adsorptive, prppfrties
(D) partition coeffhcient
87. The amperometrie titratioft- is based on the principle of
(A) diffusion current isproportional to concen tration of electroactive species
(B) diffusion current is propor¬tional to apiplied potential
(C) halfwave potential is characteristic of the substance determined
(D) half-wave potential isproportional to concen- tration of active species
88. The heterocyclic rihg present in
nizatidine is
(A) imidazole
(B) oxazole
(C) thiazole
(D) furan
89. The moist intense peak in the mass spectrum is
(A) mass peak
(B) metastable peak
(C) M+1 peak
(D) base peak
90.Whieh of the following is a taftar emetic?
(A) Potassium bitartrate
(B) antimony potassium tartrate
(C) Magnesium tartrate
(D) None of the above
91. The relationship among concen¬tration, temperature and potential ofa solution is given by
(A) IIkovic equation
(B) Henderson equation
(C) Nemst equation
(D) Van Warmeer equation
92. Tricyclic antidepressant used as antiepileptic is
(A) carbamazepine
(B) phenytoin
(C) valproate
(D) ethosuximide
93.Limit test for arenic is performed in which of the following methods?
(A) Dithizone test
(B) Gutzeit test
(C) Arsine test
(D) None of the abOve
94. Which one of the following is unsaturarted fatty acid?
(A) Linoleic acid
(B) Myristic acid
(C) Palmitic acid
(D) Stearic acid
95. SGPT is elevated in
(A) hepatitis
(B) diabetes
(C) pancreatitis
(D) None of the above
96. Erythromycin belongs to the class of
(A) macrolide
(B) Bita-lactum
(C) aminoglycoside
(D) peptide
97. Nifedipine blocks which of the following calcium channels?
(A) T-type
(B) N-type
(C) P-type
(D) L-type
98. Gold 198 is used in
(A) the treatment of pernicious anaemia
(B) the detection of tumors
(C) the treatment of rheumatoid arthritis
(D) the detection of thyroid function
99. Dialkyl diethylmalonate when treated with urea in presence of sodium ethxide gives
(A) diazepam
(B) morphine
(C) barbiturate
(D) sulfonamide
100. Prostaglandins are a group of related
(A) alcohols
(B) aldehydes
(C) fatty acids
(D) alkaloids









