 About Authors:
About Authors:
Jatin Patel*
Seth G.L. Bihani S.D. College of Technical Education,
Institute of Pharmaceutical Sciences and Drug Research,
Sri Ganganagar, Rajasthan, INDIA
*Patelj313@yahoo.com
ABSTRACT:
Thermogravimetry is a branch of physical chemistry, materials research, and thermal analysis. It is based on continuous recording of mass changes of a sample of material, as a function of a combination of temperature with time, and additionally of pressure and gas composition.
It includes different types of Thermogravimetric analysis. In this article types, Instrumentation, Procedure, Application are priscribed.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1532
INTRODUCTION
Thermal Gravimetric Analysis (TGA)
• Precisely monitoring weight loss of a sample in a given atmosphere as a function of temperature and/or time
• Atmospheres: N2, O2, air, or He
• Temperature: ambient to 1000 °C
• Records the first derivative of the mass loss

• The percent weight loss of a test sample is recorded while the sample is being heated at a uniform rate in an appropriate environment.
• The loss in weight over specific temperature ranges provides an indication of the composition of the sample, including volatiles and inert filler, as well as indications of thermal stability.
• The gas environment is pre-selected for either a thermal decomposition (inert – He or N2 gas), an oxidative decomposition (air or O2), or a combination therein. ( Beckett A.H.and Stenlake J.B.)
[adsense:468x15:2204050025]
Types of thermmogravimetric analysis Thermogravimetric analysis is usually of following types
(1) Dynamic TGA : in this type of analysis the sample is subjected to condition of continuous increase in temperature usually linear with time.
(2) Isothermal or static TGA :In this type of analysis sample is maintained at a constant temperature for a period of time during any change in weight are recorded.
(3) Quasistatic TGA: in this technique sample is heated to a constant weight at each of a series of increasing temperature.(Sharma B.K.)
Instrumentation of TGA
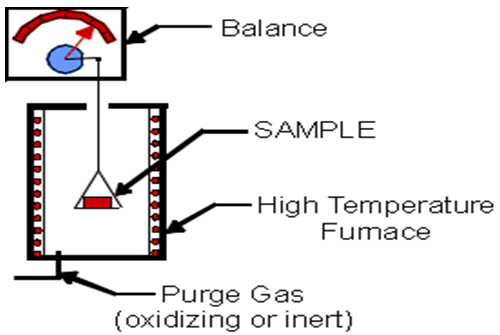
(URL instrumental)
Thermogravimetric instrumentation should include several basic components to provide the flexibility necessary for the production of useful analytical data:
a) A balance,
b) A heating device,
c) A unit for temperature measurement and control,
d) A means for automatically recording the mass and temperature changes,
e) A system to control the atmosphere around the sample
The Thermobalance
Two typical designs of the thermobalance are shown in the following:
(1) Null type: are commonly employed in these balances a sensor is employed to detect the deviation of the beam from its null point position.A restoring force either mechanical or electrical load is than applied to balance beam in order to restore it in null point position from the horizontal or vertical norm.The weight change is proportional to restoring force, which can be recorded directly by making use of transducer.
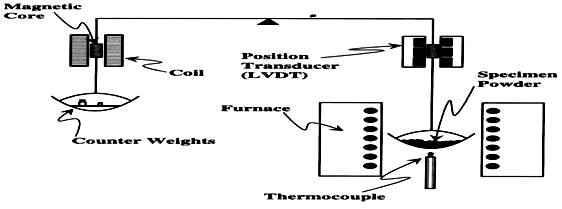
Null type
(2) Deflection type: these balances are mainly of four types
(a)Beam type: In these balances a conversion of beam deflection about the fulcrum into a suitable identifiable weight change curve take place by either of the following
(1) By photographic recorded trace
(2) By recorded signal generated by suitable displacement measuring tranceducers.
(3) By elecromechanicaly drawn curves
(b) helical type; in these type of balances an elongation or contraction takes place as a result of an elongation or contraction takes place as a result of weight change.Transducers are used for detection of weight change.Quartz is used as fiber, because it don’t gives anomalous result by change in temperature as well as fatigue problem.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
(c) cantilever beam type:in this type one end is fixed and other end is free to undergo deflection and it contains sample.
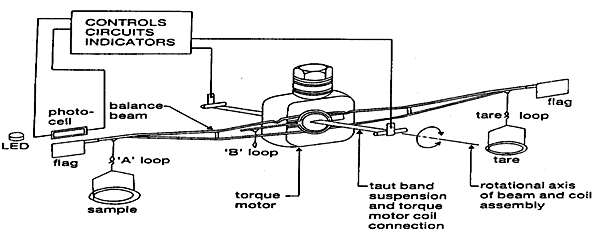
Deflection type
Deflection in beam are thus proportional to weight changes in the sample. A metallic ribbon may also be used in place of taut wire.
Deflection measurements are similar to beam type balances.
(d) Torsion balances: in these beam is attached to a taut wire acting as fulcrum a wire is firmly fixed at any end or at both ends
Balances must remain precise and accurate continuously under extreme temperature and atmosphere conditions, and should deliver a signal suitable for continuous recording
* Null-deflection weighing mechanisms are favoured in TG as they ensure that the sample remains in the same zone of the furnace irrespective of changes in mass.
* Sensitivity of balance »1mg for a 1g maximum load balance.
* The output weight signal may be differentiated electronically to give a derivative thermogravimetric curve (DTG)(Sharma B.K)
The Heating Chamber
* The furnace is normally an electrical resistive heater;
* Some basic requirements of the heating chamber are :
be non-inductively wound
be capable of reaching 100 to 200°C above the maximum desired working temperature
have a uniform hot-zone of reasonable length
reach the required starting temperature as quickly as possible
not affect the balance mechanism through radiation or convection
* In order to overcome the problem of possible temperature gradient, infrared or microwave radiation have been used in some equipment.
(a) Infrared heating: use halogen lamp, temperature up to 1400°C, heating rate can be as high as 1000°C/min, accuracy is about ±0.5°C.
(b) Microwave heating: large sample can be used because uniform heating generated within sample but temperature measurement and power control are difficult.
* The output weight signal may be differentiated electronically to give a derivative thermo gravimetric curve (DTG)
The atmosphere
Sort, pressure and flow rate of the gas in the sample chamber influence the following parameters:
• Sample reaction
Sample reactions with the gas (oxidation in the presence of oxygen).
• Heat transitions
Different heat conductivity of the gases used in an experiment.
• Buoyancy and current effects
Different density and flow rate of the gases used in an experiment.
For all thermoanalytical investigations it is very important to report the sort, the pressure and the flow rate of the gases used in the experiment.
The sample
Sample form, defect content, porosity and surface properties has influence to the behaviour on heating, e.g. single crystal sample give different response from powdered sample Large sample size cause problems like heat transfer, and gas exchange with the surrounding is reduced; in general, the use of small (~ 20 mg) specimen is preferable if sensitivity of balance permits
Sample should be powdered and spread thinly and uniformly in the container Calibration can also be done by placing a series of ferromagnetic materials in the specimen basket and a magnet below or above it, external to the furnace. When each material goes through its Curie temperature (ferro- to paramagnetic transition), a sharp ‘weight’ change will be indicated.(instrumental analysis.com)
Sample holder
The geometery shape amd sizeof a sample holder are of very importance.The size and shape of sample holder depend on the nature, weight of sample and maximum temperature range to be employed.The materials for construction of sample holders are glass, quartz alumina, stainless steel, graphite, platinum etc. sample holders are of following types.
(a) shallow type: these are used in such cases where it becomes necessary to as a weight loss. Diffusion as rate controlling step. As volatile material is produced throughout the sample mass. it should diffuse to surface instaneously to escape and be registered as a weight loss. In some cases like polymers side reactions may occur. In such cases the sample is arranged in a thin layer so that as soon as volatile fragments is formed, it is free to escape.
(b) Deep crucibles: these are used when side reactions are desired.Thes crucibles are used in industrial scale calcinations. These are also used in surface area measurements.
(c) Loosely covered crucibles; These are mainly used in self generated atmosphere studies. Wow that many of studies reported with this instrument are being done isothermically. Therefore, rate of weight loss and not exact temperature is important.
(d) Retort cups:thes are useful in boiling point studies. The retort provides the single plate of reflux essential for a single boiling point determination.(Sharma B.K.)
3. Process of TGA
A sample of material (ranging from 1 mg to 100 mg, but sometimes as large as 100 g) is placed on an arm of a recording microbalance, also called thermobalance where that arm and the sample are placed in a furnace. The furnace temperature is controlled in a pre-programmed temperature/time profile (most commonly), or in the rate-controlled mode, where the pre-programmed value of the weight changes imposes the temperature change in the way necessary to achieve and maintain the desired weight-change rate. The most common temperature profiles are: jumping to isotherm and holding there for a specified time ("soak"); temperature ramping at constant rate (linear heating or cooling); and combination of ramp and soak segments. The profile "ORTA" ("oscillation-rate thermal analysis") is used in other methods of thermal analysis, but not in TG, due to unavoidable disturbance forces. The rate-controlled method is very time-efficient, but for some types of materials it produces incorrect results or "ghost effects"; since this method does not reveal the automatically imposed temperature profile, the users may be misled by their trust for the "sophisticated, computerized program, which saves the analysis time tremendously".
(e) The gaseous environment of the sample can be: ambient air, vacuum, inert gas, oxidizing/reducing gases, corrosive gases, carburizing gases, vapors of liquids or "self-generating atmosphere". The pressure can range from high vacuum or controlled vacuum, through ambient, to elevated and high pressure; the latter is hardly practical due to strong disturbances.
(f) The commonly investigated processes are: thermal stability and decomposition, dehydration, oxidation, determination of volatile content and other compositional analysis, binder-burnout, high-temperature gas corrosion etc. The kinetic data obtained by TG are reliable only for irreversible processes, whereas reversible ones are grossly affected by diffusion, and only special procedures can handle them. Although many industrial processes could benefit from thermogravimetric investigations, the industry is often discouraged by the natural discrepancies between the data produced by milligram-size samples, and those of the bulk processes. In this respect gram-size and larger TG samples are more suitable for optimization research of industrial processes(URL instrumental analysis.com)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
4. Applications of TGA
(1) Evaluation of gravimetric precipitates: most of the important application of thermogravimetery is estimation correct drying temperature of precipitates used in gravimetric analysis.
Examples to emphasize this point
(a) Generally, triple periodate KLiFeIO6 is used for determination of lithium, however Duval found it unsuitable for gravimetric purpose because he found that TG curve of this precipitate shows that there is constant decrease in weight from 40° C to 947 °C, thus there is no drying temperature for this precipitate.
(b) The salicylodoxime method for determining zinc was rejected by Duval because he didn’t find any constant weight plateau on the TG curve . Borrel and Paris repeated the work of Duval and said that he didn’t find any constant weight plateau because he used very fast heating rate. These workers confirmed that minimum drying temperature for this precipitates is 135° C. Later Kettach confirmed that 150° C is satisfactory temperature for drying the complex, irrespective of moisture content.
(2) Evaluation of suitable standared: Duval carried out a no. of experiments on thermogravimetery to find suitable standareds in analytical chemistry.He confirmed that following substances cannot be used for preparing standared solutions.
Magnesium ammonium chloride, ammonium bicarbonate and ammonium fluoride.he observed that the following compounds are most suitable for preparing standard solution,lithium sulphate monohydrate,sodium dichromate dehydrate, sodium cobaltinitrile, hydrazinium chloride, ascorbic acid and methyl glucamine.
(3) Testing of purity of sample:as an analytical tool thermobalance can be used in determining the purity of various substance. This can be illustrated by following examples
(a) Impure sample of Calcium oxalate shows an unusual weight loss below 100° C this is not due to adsorbed water but due to some impurity.
(b) Kettach in his research paper reported how he checked the purity of samples of quicklime accidently. He was running thermogram of different samples of quick lime. Each of these TG curve showed a weightloss due to water and subsequent loss to adsorption of carbon dioxide by the original sample.After these two losses the residue of CaO doesn’t show any weight change up to 1300° C, however in one sample there occurred amarked weight loss at about 1080°C. Further examination by independent techaniques eastablished that the course of weight loss was due to presence of calcium sulphate in original sample.
(4) Curie point determination: If a ferromagnetic material is kept on a thermobalance with the pole of a magnet above the sample, the TG balance will show the weight which will be less than actual weight. Ferromagnetic materials lose their magnetism on heating at exactly reproducible temperature on curie points.A range of metas or alloys with curie point between 150° C and 1000° C is available. If suitable ferromagnetic calibration standards are placed in the sample pan of balance, and a large permanent magnet is placed below the pan,the sample will experience a downward attractions leading to an apparent increase in weight. At the curie point the loss of ferromagnetism will be reflected by an apparent loss of weight, enabling the temperature experienced by balance pan to be accurately known, using a range of standard materials, an accurate calibration curve of furnace can be obtained. However this doesn’t completely solve the problem of accurate assessment of temperature actually experienced by sample with the exception of microwave heating, heat is absorbed by exterior of sample and transferred to interior by conduction. Thus a temperature gradient will exist in sample many materials that are subject of TG investigation, will have low thermal conductivities hence this effect will be pronounced, The only remedy is to reduce the sample size to the absolute minimum.eg 10 mg and avoid rapid heating.
(5) Organic compounds: many organic compounds have been studied by DTA and TG using derivatograph
(a) When the decomposition of melonic acid was studied DTA and TG techanique, it was observed that a phase transition occurred in region of 70° C with melting at 140° C followed by decomposition at 150° C
(b) Other interesting example is study of glycine by this techanique.The compound melts with decomposition in region of 220° C. during decomposition compound glycine foams and it is therefore.difficult to prepare relatively large samples.
(c) One important eg. Is decomposition of L-glutamic acid in air.this has three clear stge of decomposition in air
(1) First pyrolidine α carboxylic acid is formed at 175° C with loss of one mole of water
(2) PACA is converted to pyrrole due to the loss of a further mole of water and one mole of CO2
(3) Total decomposition to zero weight correspond to thermal oxidation.
(6) Oxide mixture and glass technology: TG is used to study the reaction between certain oxides
(7) provide valuable information for desorption, decomposition and oxidation. e.g. dehydration of Calcium salicylate monohydrate
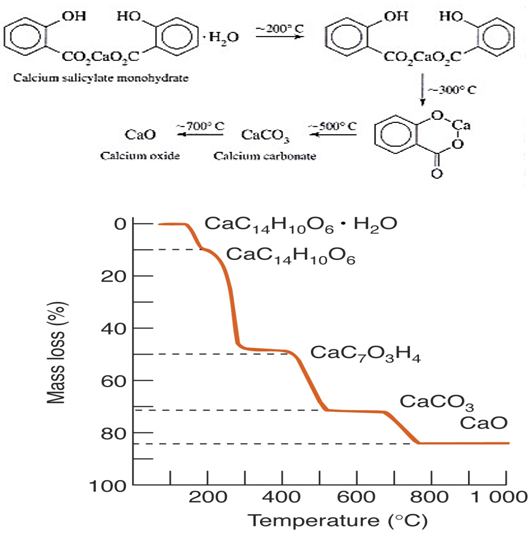
(8) knowledge of thermal stability can give information on problems like the hazards of storing explosives, shelf life of drugs, etc.
The thermal balance in a TG equipment can also be used to measure vapour pressure of a sample and magnetic susceptibility, etc.(Chatwal Gurdeep and Vogel’s Textbook of quantitative chemical analysis)
(9) Study for selection and stability of polymers
Three factors should be noted when we get a TG curve:
1. General shape,
2. The particular temperatures at which changes in mass occur (severely affected by many experimental conditions),
3. The magnitudes of the mass changes (much more use directly related to the specific stoichiometries of the reactions, independent of the many factors that affect the shape of the curves. Can be used for precise quantitative analysis).
REFERENCES
-
Willard et. al. “Instrumental methods of analysis” Seventh edition, CBS publishers and distributors, page no.767-770
-
Chatwal Gurdeep “Instrumental methods of chemical analysis” 2.703-2.716
-
Vogel’s Textbook of quantitative chemical analysis, sixth edition, page no.507-511
-
Sharma B.K. “Instrumental methods of analysis” goel publishing house page no. 234-237
-
Beckett A.H.and Stenlake J.B. “practical pharmaceutical chemistry, fourth edition-part two, CBS publishers and distributors, Daryaganj, New delhi, page no.75
-
Instrumental analysis, Thermogravimetery available at URL instrumental analysis.com/pharmaceuticals/..thermal methods./thermogravimeteryarticle_page/.../50206 (Accessed on 10-Aug-2011)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









