 About Authors:
About Authors:
Shaishavi Parikh*, Arun Parikh, Vijayalakshmi Gudaparthy
Department of pharmaceutical chemistry, L. J. Institute of Pharmacy,
Ahmedabad-382210, India.
*shaishavi21@rediffmail.com
Abstract
Thiazolo[3,2-a]pyrimidine were synthesized by the reaction of the indole aldehyde with thiourea and methyl isobuterylacetate to generate thioxo pyrimidine compound which condensed to phenacyl bromide to form thiazolo[3,2-a]pyrimidine. All synthesized compounds are characterized by the IR, Mass and NMR spectroscopy and evaluated for the antibacterial and anti fungal activity by using B. cereus, S. aureus strains of Gram positiveE. coli strain of Gram negative andC.albicans strain of fungus. The compound having nitro and methoxy substituent shows significant activity in thiazolo[3,2-a]pyrimidine derivatives.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1622
INTRODUCTION
The growing demand for biologically active compounds made multicomponent reactions attractive. The multicomponent condensation (MCC) approach is especially appealing as the products are formed in single step and the diversity can be readily achieved by varying the components. A variety of heterocyclic compounds can be readily assembled employing this approach as demonstrated by the synthesis of dihydropyrimidine using Biginelli reaction1-2.
The resulting dihydropyrimidines (DHPMs) have been reported to have antibacterial3, antiviral4, anti-inflammatory5, analgesic6, antihypertensive as well as calcium channel blocking7-8 and antioxidant9 activities. Recently, structurally simple DHPM derivative monastrol has emerged as a mitotic kinesin Eg5 motor protein inhibitor for the development of anticancer drugs10. Furthermore, the biological activity of several recently isolated marine alkaloids has also been attributed to the dihydropyrimidinone moiety in the structure. Among them the batzelladine alkaloids A and B which inhibit the binding of HIV envelope protein gp-120 to human CD4 cells are potential compounds in AIDS therapy11.
Thiazoles represent useful pharmacophore with a variety of biological activities. Various substituted thiazoles have been synthesized and examined for their antifungal and their antibacterial activity. New hybrid moieties secured by linking thiazoles with pyrimidine promises to offer fascinating scaffolds12. Thiazolo[3,2-a]pyrimidines can be prepared by the reaction between the indole aldehyde, thiourea and methyl 4-methyl-3-oxopentanoate to generate thioxo pyrimidine compound(1) which condensed to phenacyl bromide to form thiazolo[3,2-a]pyrimidine(2a-2e).
[adsense:468x15:2204050025]
EXPERIMENTAL
All the melting points were determined in open capillaries and are uncorrected. The purity of compounds was checked by TLC on silica gel G coated glass plates. IR spectra were recorded in KBr on shimadzu FT-IR 8300 spectrophotometer; 1H NMR spectra in CDCl3 on a Brucker DRX-300 at 200 MHz using TMS as an internal standard. Mass spectra were determined using direct inlet probe on a GCMS-QP2010 mass spectrometer, elemental analysis were performed on a Carlo Erba EA 1108 elemental analyser.
Synthesis of methyl 1,2,3,4-tretrahydro-4-(1H-indol-3-yl)-6-isopropyl 2-thioxopyrimidine-5-carboxylate(1)
A mixture indol-3-carboxaldehyde (1.44g, 0.01M), methyl 4-methyl-3-oxopentanoate (1.42mL, 0.012M), thiourea (1.52g, 0.020M), cupric chloride dihydrated (0.85g, 0.005M) and few drops of Conc. Hydrochloric acid was ground together for 15-20 minutes using a mortar and pastle. The initial syrupy reaction mixture solidified within 5-10 minutes. The solid was left overnight, then washed with cold water and purified and recrystallized from ethanol.
Synthesis of methyl 3-(substituted phenyl)-5-(indol-3-yl)-7-isopropyl-5H-thiazolo[3,2-a]pyrimidine-6-carboxylate(2a-2e)
A mixture of methyl 1,2,3,4-tretrahydro-4-(1H-indol-3-yl)-6-isopropyl-2-thioxopyrimidine-5-carboxylate (2.34g, 0.01M), substituted phenacylbromide (0.01M) and sodium acetate/glacial acetic acid (2g/20mL) was refluxed for 5-6 hours, the completion of reaction was monitored through TLC, the content were poured into crushed ice, the product was isolated and recrystallised from ethanol.
SCHEME
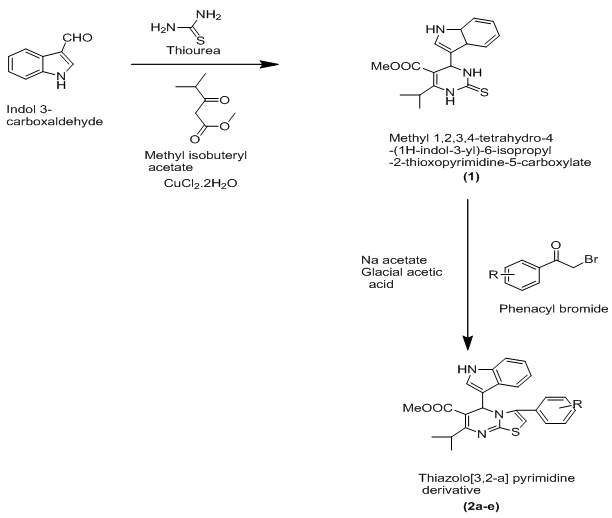
Where, R=4-Br,4-Cl,4-OH,4-NO2,4-OCH3.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
RESULT
|
|
Table 1- physical and analytical characterization data of synthesized compounds |
||||||
|
Compound |
Substituent R |
Mol. Formula |
Mol. Wt. |
m.p. (?C) |
Yield (%) |
Calcd % (found) N |
|
|
1 |
- |
C17H19N3O2S |
329.41 |
166 |
73% |
12.76(12.38) |
|
|
2a |
4-OH |
C25H23N3O3S |
445 |
196 |
68 |
9.43(9.38) |
|
|
2b |
4-NO2 |
C25H22N4O4S |
474 |
204 |
78 |
11.81(11.56) |
|
|
2c |
4-OCH3 |
C26H25N3O3S |
459 |
202 |
70 |
9.14(9.04) |
|
|
2d |
4-Cl |
C25H22ClN4O2S |
463 |
208 |
70 |
9.06(9.01) |
|
|
2e |
4-Br |
C25H22BrN3O2S |
508 |
214 |
64 |
8.26(8.18) |
|
|
|
|
|
|
|
|
|
|
|
Table-2 spectral data of all the synthesized compounds |
|||
|
Compound code |
IR |
MASS |
NMR |
|
2a |
N-H str. 3163 C-N str. 1242 -OH 3616 |
446 |
11.9 (-NH, s, 1H), 9.9 (-N-CH, s, 1H),7.1-8.1 ( Ar-H, 9H), 5.2 (-OH, s, 1H),5.1 (-CH pyr., s, 1H), 3.7 (-OCH3, s, 3H), 2.2 (-CH-, m, 1H), 1.2 (-CH3, s, 6H) |
|
2b |
N-H str. 3163 C-N str. 1242 -NO2 1296 |
475.4 |
10.5 (-NH, s, 1H), 8.7 (-N-CH, s, 1H),7.1-7.8 ( Ar-H, 9H),5.7 (-CH pyr., s, 1H), 3.9 (-OCH3, s, 3H), 2.4 (-CH-, m, 1H), 1.1 (-CH3, s, 6H) |
|
2c |
N-H str. 3178 C-N str. 1297 -OCH3 3324 |
460 |
- |
|
2d |
N-H str. 3367 C-N str. 1276 -Cl 727 |
- |
- |
|
2e |
N-H str. 3172 C-N str. 1290 -Br 482 |
- |
- |
BIOLOGICAL STUDIES
Antibacterial and antifungal activity by cup plate agar diffusion method
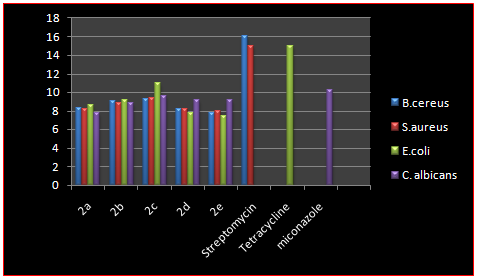
All the compounds have been evaluated for their in vitro biological assay like antibacterial activity towards gram-positive bacteria viz., B.cereus, S.aureus, and gram-negative bacteria viz.,E.coli and antifungal activity towards c. albicans at a concentration of 100 μg. The biological activities of synthesized compounds were compared with standard drugs viz., streptomycin, tetracycline for antibacterial activity and antifungal activity was compared with miconazole.
|
Compound code |
Zone of inhibition(mm) |
|||
|
Gram +ve |
Gram –ve |
Fungi |
||
|
B.cereus |
S.aureus |
E.coli |
C. albicans |
|
|
2a |
8.3 |
8.2 |
8.6 |
7.8 |
|
2b |
9.0 |
8.8 |
9.1 |
8.8 |
|
2c |
9.3 |
9.4 |
11.0 |
9.6 |
|
2d |
8.2 |
8.2 |
7.8 |
9.2 |
|
2e |
7.8 |
8.0 |
7.4 |
9.2 |
|
Streptomycin |
16 |
15 |
- |
- |
|
Tetracycline |
- |
- |
15 |
- |
|
miconazole |
- |
- |
- |
10.2 |
RESULT AND DISCUSSION
The thiazolo[3,2-a]pyrimidine derivatives have been synthesized by the synthesis of the pyrimidine thione and then it was further condensed to substituted phenacylbromide. Pyrimidine thione compound have been synthesized by the Biginelli reaction, the condensation of indole aldehyde, methylisobuteryl acetate and thiourea in presence of cupric chloride and acid as catalyst by grindstone green chemical method.
It has been observed from the antimicrobial activity data that all compounds were found to be mild to moderately active against Gram positive, Gram negative and fungal strains. However, maximum activity was observed in compounds bearing R= -OCH3 against the Gram positive strains, R= -OCH3, -NO2 against the Gram negative strain and R= -OCH3,-Cl against the fungi as compared to standard drug streptomycin for Gram positive, tetracycline for Gram negative and miconazole for the fungi.
ACKNOWLEDGEMENT
The authors are thankful to authorities of L. J. Institute of Pharmacy, Ahmadabad for providing the research facilities, Department of chemistry, Saurastra University, Rajkot for providing necessary IR spectra, Cadila Pharmaceuticals for providing the Mass spectra and IICT, Hyderabad for providing NMR spectra.
REFERENCES
1. Mohammad A, Zahra Z, “1,3-Dibromo-5,5-dimethylhydantoin as a useful reagent for efficient synthesis of 3,4-dihydropyrimidin-2-(1H)-ones under solvent-free conditions.” Chemical Papers; 2009, 63, 97–101.
2. Ezzat R, Hadi J, “A practical and green approach towards synthesis of dihydropyrimidinones: Using heteropoly acids as efficient catalysts.” Bioorganic and Medicinal Chemistry Letters; 2006, 16, 2463–2466.
3. Chitra S, Devanathan D Pandiarajan K, “Synthesis and in-vitro microbiological evaluation of novel 4-aryl-5-isopropoxycarbonyl-6-methyl-3,4-dihydropyrimidinones” European Journal of Medicinal Chemistry; 2009, 45, 1–5.
4. Kappe CO,“Biologically active dihydropyrimidones of the Biginelli type- a literature survey” European Journal of Medicinal Chemistry; 2000, 3, 1043–105.
5. Mohammad A, Sadique AJ, Harish K, “Synthesis and biological evaluation of some 4-(1H-indol-3-yl)-6-phenyl-1,2,3,4-tetrahydropyrimidin-2-ones/thiones as potent anti-inflammatory agents” Acta Pharmaceutica; 2008, 58, 467–477.
6. Chikhale RV, Bhole RP, Khedekar PB, Bhusari KP, “Synthesis and pharmacological investigation of 3-(substituted 1-phenylethanone)-4-(substituted phenyl)-1,2,3,4tetrahydropyrimidine-5-carboxylates” European Journal of Medicinal Chemistry; 2009,44, 3645–3650.
7. Zorkun I, Sarac S, Elebib S, Erol K, “Synthesis of 4-aryl-3,4-dihydropyrimidin- 2(1H)-thione derivatives as potential calcium channel blockers” Bioorganic and Medicinal Chemistry Letters; 2006, 14, 8582–8589.
8. Rovnyak GC, Atwal KS, Hedberg A, Kimball SD, Moreland S, Gougoutas JZ “Dihydropyrimidine calcium channel blockers: Basic 3-substituted-4-aryl-1,4- dihydropyrimidine-5-carboxylic acid esters as Potent antihypertensive agents.” Journal of Medicinal Chemistry; 1992, 35, 3254-3263.
9. Ismaili L, Nadaradjane A, Nicod L, Guyon C, Xicluna A, “Synthesis and antioxidant activity evaluation of new hexahydropyrimido[5,4-c]quinoline-2,5-diones and 2-thioxohexahydropyrimido[5, 4-c]quinoline-5-ones obtained by Biginelli reaction in two steps” European Journal of Medicinal Chemistry; 2008, 43, 1270-1275.
10. Bose DS, Sudharshan M Chavhan SW. “New protocol for Biginelli reaction-a practical synthesis of monastrol” Arkivoc; 2005, 228-236.
11. Snider BB Chen J. “Synthesis of Batzelladine E and its E Isomer.” Tetrahedron Letters; 1998, 39, 5697-5700.
12. Shah VR, Ghodasara JN, Patel MC, Kansagara NN, “Microwave assisted direct rapid and efficient synthesis of some novel dihydropyrimidines and evaluation of their antimicrobial activities.” International journal of chemical science; 2009, 7(3), 1575-1582.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









