 About Authors:
About Authors:
Nirav D.Langhneja1*, Tushar Vaja1, Dr. Vijay K. Parmar2
1M.Pharm, Department of Pharmaceutical Sciences,
2Associate Professor, Department of Pharmaceutical Sciences,
Sardar Patel University,
Vallabh Vidyanagar-388120, Gujarat.
*laghnejanirav@gmail.com
INTRODUCTION
Sildenafil citrate 1-[[3-(6,7-Dihydro -1-methyl- 7-oxo-3-propyl -1H-pyrazolo [4,3-d] pyrimidin-5-yl) -4-ethoxyphenyl]sulphonyl]-4-methyl piperazine citrate and it is a popularly known as Viagra .It is a compound of the pyrazolo-pyrimidinyl-methyl piperazine class, and is used to treat male erectile dysfunction (Boolell.M et.al 1996, Morales. A, et.al 1998). It is a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 inhibitor. The structural formulae is C22H30N6O4S. It is an ampholyte with pKa value 4 (pirydinium ion) and 8.8 (benzimidazole). Sildenafil citrate is twice as soluble in methanol than in water. Its solubility decreases with pH up to 9 when it starts to increase again. Sildenafil citrate could be determined by several analytical techniques, Densitometry, spectrophotometry, colorimetry, HPLC, GC-MS, MEKC and capillary electrophoresis.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1453
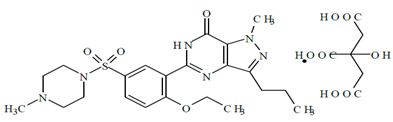
Figure 1: Structure of Sildenafil citrete.
Dapoxetine Hydrochloride, (1S)-N,N-dimethyl-3-naphthalen-1-yloxy-1-phenylpropan-1-amine hydrochloride (Figure 2) is used for treatment of premature ejaculation in men. Dapoxetine is a shortacting selective serotonin reuptake inhibitor (SSRI). SSRI's are a class of compounds typically used as antidepressants in the treatment of depression, anxiety disorders, and some personality disorders. They can also sometimes be effective and used in treating premature ejaculation problems, impotence and some cases of insomnia.Adverse events, such as nausea, dizziness, headache, diarrhoea, insomnia and upper respiratory tract infection have been reported.
[adsense:468x15:2204050025]
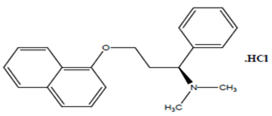
Figure 2:Structure of Dapoxetine hydrochloride.
To date, there have been no published reports about the simultaneous Quantitation of Sildenafil and Dapoxetine Hydrochloride by HPLC in bulk drug and in tablet dosage form. This present study reports for the first time simultaneous Quantitation of Sildenafil and Dapoxetine by HPLC in tablet dosage form. The proposed method is validated as per ICH guideline.
EXPERIMENTAL
Materials
Drug: Sildenafil Citrate and Dapoxetine are procured from Intas Pharma , Ahmadabad and Emcure Pharma, Pune respectively .
Chemicals and reagents used in proposed method are shown in Table 1.
Table 1:Chemicals used in present study
|
Reagent |
Grade |
Supplier |
|
Acetonitrile |
HPLC Grade |
SIGMA ALDRICH, Mumbai - india |
|
Water |
HPLC Grade |
Sisco Research Lab Pvt Ltd, Andheri ,Mumbai. |
|
Potassium dihydrogen ortho-phosphate anhydrous (KH2PO4) |
HPLC Grade |
Rankem ,RFCL Ltd, New Delhi- india |
|
Ortho-phosphoric acid (OPA) |
AR Grade |
RANCHEM, New Delhi–india |
|
Triethylamine (TEA) |
Extra pure AR |
Sisco Research Lab Pvt Ltd, Andheri ,Mumbai. |
Analytical method
Experimental conditions
The chromatographic system comprised of an Agilent 1260 series HPLC system equipped with a solvent-degasser unit and a PDA detector. The data was acquired and processed using Agilent OpenLab CDS (EZChrome edition, version A.04.02) software. Pre-filtered samples (20 µL) were injected into a ZORBAX Eclipse plus C18 column (4.6 mm I.D. × 100 mm L, 3.5 µm particle size) protected by a Agilent ZORBAX Reliance Cartridge Guard Column (4.6 mm I.D. X 12.5 mm L, 5 µm particle size packing).The mobile phase system consisted of ACN : Buffer pH 6 ( 80:20),pH was Adjusted to 6 by 0.5 % OPAand was run in isocratic mode at a flow rate was 1.0 mL/min through the column. The run time was 5 min per injection and elute was monitored at a wavelength of 220 nm.
Chromatographic method development and optimization
Initial trial experiments were conducted, in order to select a suitable mobile phase system for accurate analysis and to achieve good resolution between the two drugs. Column chemistry, solvent type, solvent strength (volume fraction of organic solvent in the mobile phase), pH of mobile phase, mobile phase additives (peak modifier), detection wavelength, and flow rate were varied to determine the chromatographic conditions giving the best separation. The suitability of the mobile phase and the flow rate was decided on the basis of system suitability parameters such as capacity factor (k’), tailing factor (Tf) and number of theoretical plates (N) and the resolution (Rs). The other factors considered are time required for analysis, ease of preparation, and use of readily available cost-effective solvents. These included Standard solution containing mixture of Sildenafil and Dapoxetine was chromatographed using mobile phase consisting of ACN: Buffer (pH 2.0, 3.0, 4.0, 5.0, 6.0) at flow rate of 1 ml/min.. The buffer of pH 6 was selected as it showed better separation between Sildenafil and Dapoxetine.The experimental work was performed in an air-conditioned room maintained at 25±1 ?C.
Preparation of stock solution and calibration standards
Accurately weighed 25 mg and 15 mg of SIL and DAPO was transferred into 25 ml volumetric flask and dissolved in water and diluted up to the mark with mobile phase to get standard stock solution containing 1000 µg/ml and 600 µg/ml of SIL and DAPO respectively. 2.5 ml of SIL and DAPO standard stock solution was diluted to 25 ml with Mobile Phase to get working standard solution containing 100 μg/ml and 60 μg/ml of SIL and DAPO.Aliquots (0.4, 0.6 ml, 0.8 ml, 1.0 ml, 1.2 ml and 1.4 ml) were withdrawn from working standard solution of SIL and DAPO mixture and diluted up to 10 ml with mobile phase to get the combined calibration standards containing 4, 6, 8, 10, 12 and 14 µg/ml of SIL and 2.4, 3.6, 4.8, 6.0, 7.2 and 8.4 µg/ml of DAPO .
System Suitability Tests
The system suitability test as per method should be performed and checked before performing any parameter (%RSD < 2%). The capacity factor, injection repeatability (n=6), tailing factor, number of theoretical plate, and resolution for the two drug peaks were the parameters tested on a combination solution containing 10 µg/ml of SIL and 6 µg/ml DAPO in order to support analytical method validation parameters like accuracy and precision.
Method Validation
Linearity
To carry out this study series of combined calibration standards of both drugs were prepared over a concentration range of 4-14 µg/ml for SIL and 2.4-8.4 µg/mlfor DAPO. The combined calibration standards were analyzed by the proposed method (n=5). The data of peak area versus drug concentration were treated by linear least square regression analysis, whereby the slope, intercept, and the correlation coefficient were determined.
Precision
The precision of the proposed method was assessed in terms of repeatability, intra-day precision, inter-day precision and reproducibility.
Repeatability
The repeatability studies were carried out by estimating response of SIL (10 μg/mL) and DAPO (6 μg/mL) six times, and results are reported in terms of % RSD.
Intra-day precision
Here, Six concentration Range (2-14 µg/ml and 2.4-8.4 µg/ml) of Standard mix of SIL and DAPO were injected three times in a single day and % RSD value was calculated to determine Intraday variation which is within limit (i.e. % RSD < 2) for both drugs.
Inter-day precision:
Here, Six concentration Range (2-14 µg/ml and 2.4-8.4 µg/ml) of Standard mix of SIL and DAPO were injected three times on different days and % RSD value was calculated to determine Intraday variation which is within limit (i.e. % RSD < 2) for both drugs.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Reproducibility :
The injection Repeatability was found to be 0.74 and 0.63 % for SIL and DAPO respectively. The overlain chromatograms of five replicate injection were shown in System Suitability.
Accuracy
Recovery studies by the standard addition method were performed in to assess the accuracy of the proposed method. Accuracy of the method was confirmed by recovery study from marketed formulation at three level of standard addition from 80 % to 120 % of label claim. The experiment was performed in triplicate. The % Recovery and RSD were calculated at every level.
Limit of detection (LOD) and limit of quantification (LOQ)
For determinations of limit of detection, the concentrations of standard lower than that of the lowest point of calibration curve were injected to the HPLC and responses were measured. The LOD value was determined by visually observing the chromatogram and considering signal to noise ratio of about 3. The LOQ was considered to be ten times the signal to noise ratio and reproducible with precision of 20 %.
Specificity and Selectivity
The retention time of SIL and DAPO were determined to check selectivity of the method. Further, the absence of interference of excipients used in pharmaceutical preparation was demonstrated. The PDA spectrum obtained from the chromatogram of the formulation mixture was compared with that of the standard drug to check specificity and peak purity analysis was also performed by OpenLab software to determine the purity of both the drug.
Robustness
The study of robustness was carried out to evaluate the influence of small but deliberate variations in the chromatographic conditions for the determination of SIL and DAPO in combined dosage form. Robustness was determined by altering pH of mobile phase, organic solvent composition and flow rate as shown in following Table 2. The average value of % RSD of the responses for determination of SIL and DAPO less than 2 % reveals the Robustness of the method.
Sample solution stability
Stability of stock solution was checked under laboratory conditions (25 ± 1 °C) and under refrigeration (8 ± 1 °C).Solution of Standard mix of SIL and DAPO (having strength of 10 µg/ml and 6 µg/ml respectively ) were kept at room temperature and in refrigerator. After 24, 48, 72, 98 and 122 hours the solutions were analyzed.
Analysis of Marketed formulation
Twenty Tablets were weighted and finely powdered. Accurately weighed tablet powder equivalent to 15 mg of DAPO and 25 mg SIL was transferred into 25 ml of volumetric flask, dissolved and diluted up to mark with mobile phase and sonicated for 30 minutes. The resulting solution was filtered using Whatman filter paper no. 41. The filtered solution (2.5 ml) was transferred to 25 ml volumetric flask and diluted with mobile phase to get a solution containing 60 μg/ml of DAPO and 100 µg/ml of SIL.The resulting solution was filtered through 0.25 µm Nylon filter and analyzed by the proposed method.
Analysis of Excipients Influence on Developed Assay Method:
Interference of excipients on recovery of the drug was studied by mixing the known amount of both the drug with excipients and then recovery was measured. Effect of the excipient on chromatogram was also observed.
Calculations and Statistical Analysis
Standard regression curve analysis was computed using EXCEL® software (Microsoft Corporation, USA) without forcing through zero. Means, standard deviation, were also calculated using the same software.
RESULTS AND DISCUSSION
Chromatographic Method Development and Optimization
For better resolution between both the drugs different mobile phases were tried on a preselected column that is ZORBAX Eclipse plus C18 column (4.6 mm I.D. × 100 mm L, 3.5 µm particle size). The detection of eluted peaks was done by a PDA detector. The solvent type, solvent strength (volume fraction of organic solvent(s) in the mobile phase), detection wavelength, and flow rate were varied to determine the chromatographic conditions giving the best separation. The pH of mobile phase majorly affects the separation of the compound.The buffer of pH 6 was selected as it showed better separation between SIL and DAPO .Selected column has also capacity to withstand at this acidic pH. Increase in acetonitrile content in mobile phase reduce retention time of Ibuprofen but has no much effect on Famotidine, so proportion of acetonitrile is kept optimum to get best resolution and peak shape. Optimized mobile phase is acetonitrile: water (80:20 v/v), pH 6 adjusted with 0.5% ortho-phosphoric acid. The UV spectra of the analytes were determined independently and in combination. Flow rate is kept 1.0 ml/min while detection wavelength is kept 220 nm which gives best peak purity and shape. The mobile phase composition acetonitrile: water with pH 6 adjusted with 0.5% ortho-phosphoric acid in the ratio 80:20 % v/v, flow rate of 1 ml/min and detection wavelength of 220 nm gives good separation with fulfilling all the requirements of system suitability parameters and single run can be completed within 5 minutes. Retention time of SIL and DAPO were obtained 1.55 and 3.88 min respectively with resolution of 4.51 between both the drugs
System suitability tests
The results of system suitability tests assure feasibility and adequacy of the proposed method for simultaneous estimation of the two drugs in pharmaceutical formulation. The results of system suitability tests are shown in Table 2.%RSD for SIL and DAPO respectively was found to be 0.74 and 0.63.
Table 2: System Suitability data
|
PARAMETER |
SIL |
DAPO |
|
Retention time(Rt in min) |
1.55 |
3.88 |
|
capacity factor (k) |
1.22 |
4.58 |
|
Resolution# (Rs) |
0 |
4.51 |
|
Tailing factor (Tf) |
1.33 |
1.25 |
|
Inj. Repeatability* (RSD) |
0.74 |
0.63 |
|
No. of Theo Plates (N) |
3858 |
2447 |
|
Width (W) |
0.34 |
0.69 |
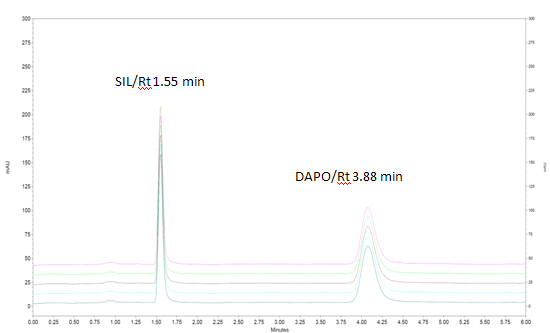
Figure 3: Chromatogram of Standard solution containing 10µg/ml of SIL and 6µg/ml DAPO using ACN:Buffer(80:20 pH 6) as mobile Phase for System Suitability.
Method Validation
Linearity
The linearity range for both the drugs was selected on the basis of the ratio of SIL and DAPO in dosage form, which is 50:30.The linearity for SIL and DAPO was found in the range of 4-14 µg/ml and 2.4-8.4 µg/ml respectively is shown in overlay chromatogram of SIL and DAPO, figure 4. Calibration curve of SIL and DAPO is presented in figure 5 and 6 respectively. Correlation coefficient, linearity equations (slope and intercept) are presented in table 3.
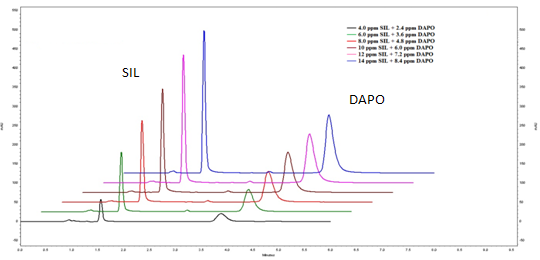
Figure 4: Overlay chromatogram
Table 3: Data For Lineariry
|
Parameter for |
SIL |
DAPO |
|
Linearity range |
2-14 µg/ml |
2.4-8.4 µg/ml |
|
Linearity equation |
y = 22323x – 27743 |
y = 45705x – 50480 |
|
Correlation co-efficient |
0.997 |
0.996 |
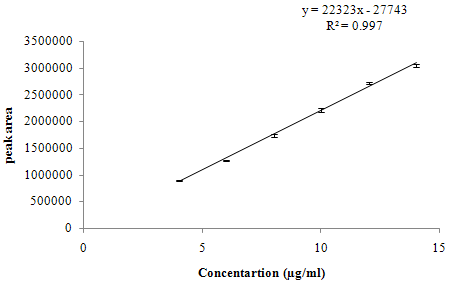
Figure 5: Calibration curve of SIL.
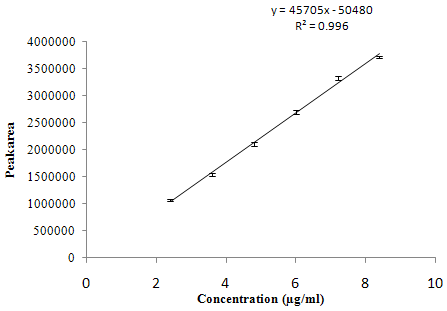
Figure 6: Calibration curve of DAPO.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Precision
Intraday precision
Intra-day precision was determined at three concentration levels injected 3 times a day (n=9) at 2 hours interval and it showed good repeatability with % RSD less than 2. Six concentration Range (2-14 µg/ml and 2.4-8.4 µg/ml) of Standard mix of SIL and DAPO were injected three times in a single day and % RSD value was calculated to determine Intraday variation which is within limit (i.e. % RSD < 2) for both drugs.
From the data of Intraday precision %RSD is 0.82-1.78 for SIL and 0.73-1.88 %RSD for DAPO .
Interday precision
Inter-day precision was determined at three concentration levels injected 3 times a in 3 day (n=9) and it showed good repeatability with % RSD less than 2. Six concentration Range (2-14 µg/ml and 2.4-8.4 µg/ml) of Standard mix of SIL and DAPO were injected three times on different days and % RSD value was calculated to determine Interday variation which is within limit (i.e. % RSD < 2) for both drugs.
From the data of Interday precision %RSD is 0.23-1.96 for SIL ,0.30-1.44 for DAPO.
c)Injection Repeatability:
The injection Repeatability was found to be 0.74 and 0.63 % for SIL and DAPO respectively. The overlain chromatograms of five replicate injection were shown in Sys. Suitability.
Accuracy
Accuracy of the method was confirmed by recovery study from marketed formulation at three level of standard addition from 80 % ,100% and 120 % of label claim.
Table 4: Accuracy Data for SIL and DAPO *(n=3)
|
Level |
Amt. of sample (Formulation) µg/ml * |
Amt. of Standard drug added µg/ml * |
Amt. recovered |
% Recovery ± %SD |
%RSD |
|||||
|
|
||||||||||
|
SIL |
DAPO |
SIL |
DAPO |
SIL |
DAPO |
SIL |
DAPO |
SIL |
DAPO |
|
|
0 % |
6 |
3.6 |
0 |
0 |
5.99 |
3.6 |
99.87± 0.74 |
100 ± 0.45 |
0.74 |
0.45 |
|
80 % |
6 |
3.6 |
3.6 |
2.16 |
10.70 |
6.44 |
99.16 ± 1.07 |
99.44 ± 0.54 |
1.08 |
0.55 |
|
100 % |
6 |
3.6 |
6 |
3.6 |
11.88 |
7.19 |
99.04 ± 0.68 |
99.98 ± 0.84 |
0.68 |
0.84 |
|
120 % |
6 |
3.6 |
7.2 |
4.32 |
13.03 |
7.85 |
98.73 ± 1.65 |
99.23 ± 0.20 |
1.67 |
0.20 |
Limit of Detection (LOD) and Limit of Quantitation (LOQ)
The LOD as calculated by standard formula was found to be 0.83 μg/ml and 1.19μg/ml for SIL and DAPO, respectively.
The LOQ as calculated by standard formula was found to be 2.52 μg/ml and 3.60 μg/ml for SIL and DAPO, respectively.
Specificity and Selectivity
The selectivity of the method is depicted by the two sharp well resolved peaks for SIL and DAPO obtained at their respective retention times, i.e., 1.55 and 3.92 min, respectively. The specificity of the method was assessed by comparing chromatograms obtained from drug standards (Figure 7) with that obtained from tablet solutions (Figure 8). The retention times of the drug standards and the drugs from sample solutions were identical, confirming the specificity of the method.
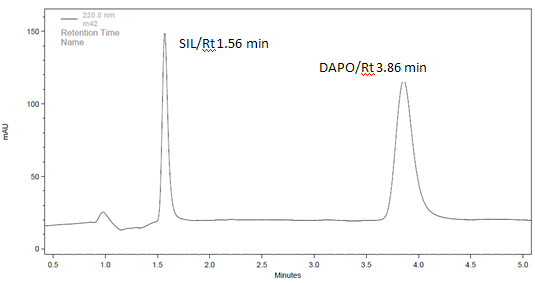
Figure 7: Chromatogram of Standard solution containing 10µg/ml of SIL and 6µg/ml DAPO using ACN:Buffer (80:20 pH 6) as mobile Phase.
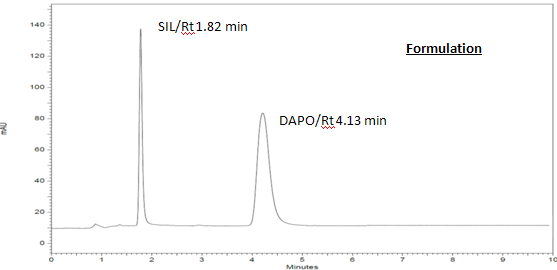
Figure 8: Chromatogram of 10 µg/ml SIL and 60 µg/ml DAPO of Marketed Formulation DaSutra (Hetro Pharma) using ACN:Buffer (80:20 pH 6) as mobile Phase.
Robustness
The main affecting parameters like flow rate, mobile phase composition and pH of the mobile phase were varied at 5% level and it showed no significant changes in retention time, peak shape, capacity factor and resolution of the two drugs. The area of the peak was not affected significantly by these variations. These experiments assured capability and robustness of the proposed method.
Table 5: Result For Robustness.
|
Drug |
Parameter |
% Change in Parameter |
Area |
Mean |
SD |
%RSD |
|
|
pH |
+5% |
2153993 |
2175553.06 |
19876.44 |
0.91 |
|
|
0% |
2193148.2 |
||||
|
SIL |
-5% |
2179518 |
||||
|
|
Flow rate |
+5 |
2109895 |
2153207.40 |
41728.88 |
1.93 |
|
|
0% |
2193148.2 |
||||
|
|
-5% |
2156579 |
||||
|
|
Mobile Phase |
+5% |
2158146 |
2158437.73 |
34565.52 |
1.60 |
|
|
0% |
2193148.2 |
||||
|
|
-5% |
2124019 |
||||
|
|
pH |
+5% |
2740266 |
2743543.80 |
34137.52 |
1.24 |
|
|
0% |
2711163.4 |
||||
|
DAPO |
-5% |
2779202 |
||||
|
|
Flow rate |
+5 |
2759846 |
2709102.46 |
51804.75 |
1.91 |
|
|
0% |
2711163.4 |
||||
|
|
-5% |
2656298 |
||||
|
|
Mobile Phase |
+5% |
2664535 |
2676812.13 |
30149.66 |
1.12 |
|
|
0% |
2711163.4 |
||||
|
|
-5% |
2654738 |
Sample Solution Stability
Solution of Standard mix of SIL and DAPO (having strength of 10 µg/ml and 6 µg/ml respectively), stored under refrigerated condition (8±1?C) and at room temperature (25±1?C), combination working solutions were made after suitable dilution with the solvent, on each day of analysis. There was no significant change in analyte composition until period of 5 days. The mean RSD between peak areas, for the samples stored under both refrigeration and ambient conditions was found to be less than 3%, suggesting that the individual drug solution can be stored without any degradation for the time interval studied.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.









