 About Author: Debashish Satpathy
About Author: Debashish Satpathy
Roland Institute of Pharmaceutical Sciences
Berhampur
Odisha
Protease is any enzyme that conducts proteolysis by hydrolysis of the peptide bonds that link amino acids together in the polypeptide chain. They are also called proteolytic enzymes or proteinases.
Classification Proteases are currently classified into six groups:
Serine proteases
Threonine proteases
Cysteine proteases
Aspartic acid proteases
Metalloproteases
Glutamic acid proteases
[adsense:336x280:8701650588]
The threonine and glutamic acid proteases were not described until 1995 and 2004, respectively. The mechanism used to cleave a peptide bond involves making an amino acid residue that has the cysteine and threonine (peptidases) or a water molecule (aspartic acid, metallo- and glutamic acid peptidases) nucleophilic so that it can attack the peptide carbonyl group. One way to make a nucleophile is by a catalytic triad, where a histidine residue is used to activate serine, cysteine, or threonine as a nucleophile.
Reference ID: PHARMATUTOR-ART-1110
Occurrence
Proteases occur naturally in all organisms. These enzymes are involved in a multitude of physiological reactions from simple digestion of food proteins to highly-regulated cascades (e.g., the blood-clotting cascade, the complement system, apoptosis pathways, and the invertebrate prophenoloxidase-activating cascade). Peptidases can either break specific peptide bonds (limited proteolysis), depending on the amino acid sequence of a protein, or break down a complete peptide to amino acids (unlimited proteolysis). The activity can be a destructive change, abolishing a protein's function or digesting it to its principal components; it can be an activation of a function, or it can be a signal in a signaling pathway. Bacterium also secretes proteases to hydrolyse the peptide bonds in proteins and therefore break the proteins down into their constituent monomers.Proteases are also a type of exotoxin, which is virulence factor in bacteria pathogenesis. Bacteria exotoxic proteases destroy extracellular structures. Protease enzymes are also found used extensively in the bread industry in Bread improver.
Proteases are involved in digesting long protein chains into short fragments, splitting the peptide bonds that link amino acid residues. Some of them can detach the terminal amino acids from the protein chain (exopeptidases, such as aminopeptidases, carboxypeptidase A); the others attack internal peptide bonds of a protein (endopeptidases, such as trypsin, chymotrypsin, pepsin, papain, elastase).
Proteases are used throughout an organism for various metabolic processes. Acid proteases secreted into the stomach (such as pepsin) and serine proteases present in duodeum (trypsin and chymotrypsin) enable us to digest the protein in food; proteases present in blood serum (thrombin, plasmin, Hageman factor, etc.) play important role in blood-clotting, as well as lysis of the clots, and the correct action of the immune system. Other proteases are present in leukocytes (elastase, cathepsin G) and play several different roles in metabolic control. Proteases determine the lifetime of other proteins playing important physiological role like hormones, antibodies, or other enzymes -- this is one of the fastest "switching on" and "switching off" regulatory mechanisms in the physiology of an organism. By complex cooperative action the proteases may proceed as cascade reactions, which result in rapid and efficient amplification of an organism's response to a physiological signal.
Proteolytic enzymes are ubiquitous in occurrence, being found in all living organisms, and are essential for cell growth and differentiation. The extracellular proteases are of commercial value and find multiple applications in various industrial sectors. Although there are many microbial sources available for producing proteases, only a few are recognized as commercial producers. A good number of bacterial alkaline proteases are commercially available, such as subtilis in Carlsberg, subtilis in BPN' and Savinase, with their major application as detergent enzymes. However, mutations have led to newer protease preparations with improved catalytic efficiency and better stability towards temperature, oxidizing agents and changing wash conditions.
Sources of bacterial proteases
Protease production is an inherent capacity of all microorganisms and a large number of microbes belonging to bacteria, fungi, yeast and actinomycete are known to produce proteases. Bacteria are the predominant group of alkaline protease producers, the genus Bacillus being the most common source (Gupta et al., 2002 b). Some of the potential alkaline protease producing bacilli are strains of B. lichiniformis, B. subtilis and B. amyloliquifaciens. Pseudomonas, Flavobacterium, Halobacterium, Vibrio, Serratia, Staphylococcus, Brevibacterium, Alcaligenes have also been explored for protease production (Gupta et al., 2005). Among actinomycetes, strains of Streptomyces, Nocardia and Nocardiopsis are potential ones. In fungi, aspergili is the most exploited group and the strains of Neurospora, Penicillium, Ophiostoma, Myxococcus, Rhizopus etc. are common producers of proteases (Gupta et al., 2005). Only those microbes that produce substantial amounts of extracellular enzyme are of industrial importance. Several products based on bacterial proteases have been launched successfully in the market in past few years
Applications
Proteases have considerable application in the detergent, leather tanning and food industries (Kelly and Fogarty, 1976; Godfrey and Reichelt, 1985). At present a large proportion of commercial proteases are derived from neutrophilic Bacillus species. Different alkaline protease producing alkaliphilic microbial strains were isolated in many laboratories (Horikoshi and Akiba, 1982; Durham et al., 1987; Takami et al., 1989; Yum et al., 1994). Alkaline proteases produced by alkaliphilic strains showed better resistance to alkali and some other denaturing chemicals in the reaction mixture and have higher affinity towards proteinaceous substrates, properties which are very important for application in the detergent and leather tanning industries (Kalisz, 1988). This has given the impetus for the continuous search of alkaliphiles producing novel alkaline proteases. Naturally occurring alkaline habitats are found in different parts of the world. Isolation and screening of alkaliphiles from such habitats is expected to give alkaline protease producing microorganisms potentially useful for many applications.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Industrial applications of proteases
The specific applications of proteases in different industries are reviewed in brief in the following sections:
(a) Food and feed industry
The food processing industry is highly dependent on enzymes and during processing of food, enzymes are added to modify or improve the characteristics. Food technologists are constantly improving enzyme technology by selecting those enzymes (protease) which can improve one particular unit operation of food production e.g. substituting fish protein hydrolysate for milk in place of calf milk (Diaz-Casteneda and Brisson, 1989), saving energy in production processes (Christensen, 1989), extracting juice (Kilara, 1982) and modifying the functional properties of proteins (Alder-Nissen et al., 1983).
Proteases are used in baking industry for hydrolysis of gluten for quick dough preparation. The proteases breakdown the gluten present in the raw flour whose high content is undesirable for the production of biscuits and cookies. Dairy is one of the important food industries where enzymes have been used since prehistoric time for processing of milk. In the beginning, the dairy industry used to employ rennet (chymosin) from the calf stomach for the manufacture of cheese (Chaplin and Bucke, 1990). Use of animal rennet has now been replaced by proteases from microbial sources (Gerhartz, 1990). In brewing industry, proteins are used to hydrolyse the cereal mash proteins so that the yeast can assimilate the peptides and amino acids during the fermentation. Protease is added to the cereal mash to increase the amount of free amino nitrogen and also during the beer finishing stage to remove the chill haze from beer (Ward, 1983).
(b) Leather industry
The conventional methods in leather processing involve the use of hydrogen sulfide and other chemicals, creating environmental pollution and safety hazards. Thus, for environmental reasons the biotreatment of leather using an enzymatic approach is preferable as it offers several advantages, e.g. easy control, speed and waste reduction, thus being ecofriendly (Anderson, 1998). Proteases find their use in the soaking, dehairing and bating stages of preparing skins and hides. The enzymatic treatment destroys undesirable pigments, increases the skin area and thereby clean hide is produced. George et al. (1995) used B. amyloliquefaciens alkaline protease for unhairing hides and skins. Hameed et al. (1999) used B. subtilis K2 alkaline protease in bating and leather processing. (c)
(c) Medical usage
Proteases are also used for developing products of medical importance. Kudrya and Simonenko, 1994 exploited the elastolytic activity of B. subtilis 316M for the treatment of burns, purulent wounds, carbuncles and deep abscesses. Kim et al. (1996) reported the use of alkaline protease from Bacillus sp. strain CK 11-4 as a thrombolytic agent having fribrinolytic activity. Oral administration of proteases from Aspergillus oryzae has been used as a diagnostic aid to correct certain lytic enzyme deficiency syndromes (Rao et al., 1998).
(d) Detergent Industry
Proteases find the largest single application in laundry detergents. Proteases are present in different brands of detergents for use in home and commercial establishments. Proteases have been added to laundry detergents for over 50 years to facilitate the release of proteinaceous materials in stains such as those of milk and blood. Detergents such as Era Plus (Procter and Gamble), Tide (Colgate Palmolive) and Dynamo (Procter and Gamble) contain proteolytic enzymes, the majority of which are produced by the members of the genus Bacillus (Samal et al., 1989).
Literature surveyed
International Status
Folasade M. OLAJUYIGBE et al, screened and isolated proteolytic bacteria from soil samples of Ikogosi warm spring (SW, Nigeria) and studied Production dynamics of extracellular protease from Bacillus species. Eighteen isolates were positive on skim milk agar (10%) of which fifteen produced protease in culture broth. Three isolates, identified as Bacillus macerans IKBM-11, B. licheniformis IKBL-17 and B. subtilis IKBS-10, were selected for further study. These Bacillus species could grow up to 65°C within a broad pH range of 5 to 10 with an optimal growth temperature and pH at 60°C and 8.0, respectively. For the three Bacillus species, protease production occurred between 37°C and 65°C and pH 5 to 10. Maximum growth and maximum enzyme production was observed at 48 h when grown in 50 ml medium (pH 8.0) under shaking condition at 60°C. The results showed that Bacillus species under study are good producers of extracellular protease at high temperature. This might be an indication that proteases produced would be thermostable.
Jyothi Bezawada et al., reported Protease Producing bacteria from Quebec Soil and Water Sample Seventy-four bacterial isolates were recovered from 10 protein rich soil and water samples collected from Quebec City. On the basis of colony size, texture, and microscopic characteristics, the isolates were categorized into 33 types. Among these, five isolates showed good proteolytic activity (>5), eight isolates showed moderate activity (between >3.0 and <5.0), 12 isolates showed poor activity (between >2.0 and <3.0), and eight isolates exhibited very poor activity (below 2.0). The five promising isolates were identified as Bacillus strains, which produced higher amount of protease and maximum cell concentration in fermentor than a shake flask in a shorter time. The enzyme produced by five strains of Bacillus showed similar thermal activity and stability. The proteases retained 31 and 10% of their original activity after 2 h at 50° and 60°C, respectively, but lost all activity at 70°C. However, the optimum pH and pH stability of proteases was from 6.0 to 9.0 and 6.0 to 8.0, respectively. The enzyme activities were accelerated by the addition of Mg2+, Ca2+, Mn2+, Fe2+, Co2+, Cu2+, and Na+, whereas these were inhibited by Hg2+ and EDTA. Thus, all evaluated isolates were suitable for the production of protease.
Franz Schinner et al., reported extracellular protease producing psychotrophic bacteria from high alpine habitats. They isolated Four hundred and thirty psychotropic strains from high alpine environments of the western and eastern Alps in Europe. Of the isolates, 77% were bacteria, 5% among them were actinomycetes. 20% of the isolates were yeasts, and 3% were hypomycetes. All bacterial strains, with the exception of actinomycetes, were tested for their optimum growth temperature and screened for the production of extracellular proteases. The optimum temperature for the growth of the bacterial strains was within the range of 10 to 25 0C. Almost half of the bacterial strains excreted protease into the medium at a cultivation temperature of 10 0C. The major part of the cell-free-protease- containing culture filtrates showed a maximum caseinolytic activity at pH 7 and 30 0 C. Sensitive to EDTA indicates that most bacteria produced metalloproteases. Fifty four producers of protease were selected for taxonomic characterization. The genus Pseudomonas, especially the P. fluorescens and P.pausimobilis, was found to be dominating. Jong-Wha Lee and Kyung-Sook Whang Isolated and Characterised alkaline Protease Producing Oligotrophic Bacteria from Serpentinite Soil.
National Status
Srinivasan et al., isolated and characterized thermostable protease producing bacteria from tannery industrial effluent. Among nine protease producing strains screened, one was selected as promising thermostable protease producer and identified as Bacillus sp. The activity of the protease produced by this organism is stable up to 700C. The optimum yield was achieved after 48 hours of culture, at 600C with the pH 8.0. The desired protein was precipitated from the crude extract by using ammonium sulfate (70%) followed by dialysis and purified by Ion-exchange chromatography. The maximum protease activity was observed at 650C and at pH 8.0.
Kunamneni Adinarayana et al., investigated the effect of Bacillus subtilis PE-11 cells immobilized in various matrices, such as calcium alginate, k-Carrageenan, ployacrylamide, agar-agar, and gelatin, for the production of alkaline protease. Calcium alginate was found to be an effective and suitable matrix for higher alkaline protease productivity compared to the other matrices studied. All the matrices were selected for repeated batch fermentation. The average specific volumetric productivity with calcium alginate was 15.11 U/mL/hour, which was 79.03% higher production over the conventional free-cell fermentation. Similarly, the specific volumetric productivity by repeated batch fermentation was 13.68 U/mL/hour with k-Carrageenan, 12.44 U/mL/hour with agar-agar, 11.71 U/mL/hour with polyacrylamide, and 10.32 U/mL/hour with gelatin. In the repeated batch fermentations of the shake flasks, an optimum level of enzyme was maintained for 9 days using calcium alginate immobilized cells. From the results, it is concluded that the immobilized cells of B subtilis PE-11 in calcium alginate are more efficient for the production of alkaline protease with repeated batch fermentation. The alginate immobilized cells of B subtilis PE-11 can be proposed as an effective biocatalyst for repeated usage for maximum production of alkaline protease.
PLAN OF WORK
Protease enzyme has found a wide range of application in pharmaceutical biotechnologies, in industries and medicines. Though extensive work has been done on protease still more research works are going on. Encouraged by this observation, this present experimental study is conducted to screen and isolate such protease producing bacteria from a kitchen exhaust, which could have wide industrial application.
Material and methodology
Collection of samples:-
Kitchen exhaust samples were collected from the kitchen of hostels and transported to laboratory in sterile containers.
Isolation and Screening of protease producing bacteria from sample by various methods:-
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
SERIAL DILUTION METHODS:
It is the simplest and most widely used method .In this method, the original inoculums is subjected to serial successive dilutions, so that the concentration of the microbes in a fixed quantity of liquid gradually becomes less and less. When these serial dilutions are plated, colonies will appear discrete and far removed from one another.
The principle of serial dilution may be explained as follows:-
If we prepare a 10 ml sample of water or soil or suspension and assume that it contains 1000 microbes; then each ml of this solution consist 100 microbes. Take 1 ml of this and add 9ml of sterile distilled water to make the volume to 10 ml. This dilution will have 1:10 or 100 microbes per 10 ml or 10 microbes per 1 ml. This may be called 10-1 concentration of the original sample. From this dilution if we take 1 ml and add 9ml of distilled water to make it to 10 ml, then each ml will have 1 microbe. This concentration is 1:100 or 10-2 of the original concentration. This dilution of one microbe per ml is the starting point of pure culture. Further culturing will yield microbes of one type: otherwise known as pure culture.
SPREAD PLATE METHOD:-
The medium was poured in four Petri plates and allowed to solidify. After getting solidified an aliquot of the inoculums was placed on the medium surface (in Petri plate) and is spread uniformly with a bent sterile glass rod. This helps in diluting the cell concentration in the inoculums. By this method cell aggregates gets separated .When colonies develop, they may be picked up and further spread on fresh medium or may be taken for streak plate method.
STREAK PLATE METHOD:-
The colonies developed from the spread plate method was picked up and placed on the solid agar medium (in Petri plate) with the help of inoculation loop. From the point of origin, thin and gentle lines of the loop are drawn over the surface of the medium.
The purpose of streaking is to thin out the inoculums successively so that the microbes gets separated In the beginning of the streak, microbes are crowded and colonies develop closely ; but as streaking proceeds cells gets separated as the needle contains fewer and fewer cells . Hence at the last stretch of the streak, few well developed and clearly separated colonies develop. It will be thus to obtain pure cultures.
PRELIMINARY SCREENING:-
Isolation of Protease producing bacteria from Kitchen extract on Starch casein agar
Starch casein agar weighing 6.3 grams were taken in 100 ml distilled water, heated until a clear solution was found. After this the clear solution was transferred into a conical flask and was autoclaved to render it sterile. Then it was poured into sterilized petri plates in laminar air flow chamber and left to be solidified.
After this the pure bacterial culture was streaked horizontally on the solidified starch casein agar medium with the help of inoculum loop. This was then kept in the BOD incubator at 37 0C for 24-48 hours, which showed significant bacterial growth. A hydrolysis zone was found around the pure culture.
Gram staining AIM: -
To perform the gram staining procedure to differentiate the bacteria into gram positive and gram negative groups.
INTRODUCTION: An important bacteriogical differential staining procedure was discovered by Dr. Hans Gram in 1884. The ability of bacteria to either retail or lose the stain generally reflex fundamental structural differences in the cell wall and is an important taxonomic feature. Hence gram stain is used as an initial step in identification of bacteria. This staining technique helps to determine gross morphology of the bacteria.
Differentiation of bacteria into two groups:-
Gram positive bacteria
Gran negative bacteria
PRINCIPLE: The explanation for “gram reaction” is associated with structure and composition of the cell wall. Cell walls of gram negative bacteria are generally thinner than those of gram positive bacteria.
Gram positive bacteria are made up of 40-80% by dry weight of peptidoglycan and lower lipid content. Gram negative bacterial cell wall contains a lower % of peptidoglycan about 1-10% by dry weight and higher percentage of lipid.
During staining of gram negative bacteria, the alcohol treatment extracts the lipid which results in increased porosity or permeability of the cell wall. T he pores in the peptidoglycan in gram negative bacteria remains sufficiently large even after alcohol treatment which allows the crystal violet sample to be extracted . Thus the Gram negative organism gets decolorized. These cells subsequently take the color of the container stain-saffranin
The cell wall of gram positive bacteria because of lower lipid content, becomes dehydrated during the treatment of alcohol. The pore size decreases, permeability is reduced and crystal-violet iodine complex is formed which cannot be extracted. These cells thus remain purple.
MATERIALS REQUIRED:-
Cultures: 24 hrs culture of bacteria
Reagent: crystal violet stain, gram’s iodine, gram’s alcohol, saffranin stain.
Equipment: Bunsen burner, inoculation loop, glass slides, microscope, blotting paper and staining rack.
PROCEDURE: A thin smear of the given bacterial culture is made with a loopfull of water taken on a slide. The smear is air dried and heat fixed. The fixed smear is covered with crystal violet stain for one minute and then washed carefully with water. On observing the slide on oil immersion objective of compound microscope all cells appear purple in colour.
Gram’s iodine is added on the smear and is left for one minute and then drained off and washed with water. All cells still appear purple in colour.
The smear is then decolourised using gram’s alcohol for about 45 seconds (till purple stain just stops coming off the slide). Gently wash the slide under running tap water and drained completely.
The smear is now counter with saffranin for about 30 seconds and later drained off with water. The decolourised cells take up the colour of the saffranin and appear pink in colour.
The slide is blotted dry and examined under oil immersion objective.
Biochemical Characterisation:
The biochemical identification of the potential isolate was made by using KB002 HiAssorted TM biochemical test kit. The biochemical test kit was procured from Himedia Laboratories Pvt. Ltd., Mumbai.
All the biochemical tests performed by KB002 HiAssorted Biochemical test kit of Himedia Laboratories, Indole, Methyl Red and voges-Proskauer test , Starch, and Gelatin liquefaction as per Conn et al., 1957 Catalase Smibert and Krieg, 1994, Oxidase Tarrand and Groschel 1982, Lipid , Casein hydrolysis tests Cappuccino and Sherman 2007 analysed accordingly.
Fermentation of carbohydrates
Fermentative degradation of various carbohydrates such as glucose, adonitol, lactose, arabinose, sorbitol by microbes under anaerobic conditions was carried out on fermentation broth. The fermentation broth contains ingredients of nutrient broth, a specific carbohydrate and a pH indicator phenol red which is red at neutral pH and turns yellow at or below a pH of 6.8 due to the production of an organic acid. The isolate which is able to catabolize carbohydrates changes the medium colour to yellow due to acid production.
Gelatin liquefaction
All the isolates were inoculated into nutrient gelatin deep tubes and incubated for 48 h. Then the cultures were placed in a refrigerator at 4°C for 30 min. Cultures that remain liquefied produce gelatinase and indicate the rapid gelatin hydrolysis.
Nitrate reduction
Isolates were inoculated in Nitrate broth medium (Nutrient broth supplemented with 0.1 % KNO3).After 24 h incubation solution A (Sulfanilic acid) followed by solution B (α- naphthylamine) was added to nitrate broth cultures. The immediate development of cherry red colour due to formation of coloured Sulfobenzene azo- α- naphthylamine (red) is an indicative of NO3- reduction.
IMViC Tests
The IMViC tests consist of four different tests: i) indole production; ii) methyl red; iii) Voges-Proskauer; and iv) Citrate utilization.
Indole test
All the isolates were inoculated on tryptone broth and incubated at 48°C for 24 h. Formation of cherry-red colour in the top layer of the tube by the addition of kovac’s reagent (dimethylaminobenzaldehyde) is a positive test for indole production. Tryptophan, an essential amino acid is oxidized by some microorganisms by the enzyme tryptophanase resulting in the formation of indole, pyruvic acid and ammonia.
Methyl-Red and voges-Proskauer test
Isolates were inoculated into MRVP broth tubes and incubated at 48°C for 48 h. Methyl red indicator was added to MR broth tubes after incubation. The methyl red indicator in the pH range of 4 will remain red throughout tube which is indicative of a positive test, while turning of methyl red to yellow is a negative test. V-P reagent I and V-P reagent II were added to V-P broth tubes. The development of crimson-to ruby pink (red) colour is indicator of positive VP test while no change in coloration is a negative test.
Citrate utilization test
Isolates were streaked on Simmon’s citrate agar medium and incubated at 48°C for 48 h. Simmon’s citrate agar is an organic synthetic medium where sodium citrate is the only source of carbon and energy. Bromothymol blue is used as an indicator. When the citric acid is metabolized the CO2 generated combines with sodium and water to form sodium carbonate an alkaline product which changes the colour of the indicator from green to blue and this indicates a positive test.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Hydrogen sulfide production Test
Isolates were grown on SIM agar medium for 24 h at 48°C. Blackening of the medium indicates positive test. This black colour is due to the production of H2S from an ingredient of the medium (i.e. sodium thiosulfate) that then combines with another ingredient of the medium ( ferrous ammonium sulfate) resulting in the formation of the black insoluble compound, ferrous sulfide.
Catalase Test
Isolates were grown on trypticase soya agar for 48 hrs at 48°C. 3 % hydrogen peroxide was poured onto the colonies. Formation of air bubbles indicates the presence of catalase enzyme.
Urease Test
Isolates were grown on urea agar medium for 24 h at 48°C. Appearence of deep pink colour due to ammonia production is evidence of urease production by the microorganism. The urea agar medium contains the pH indicator phenol red which changes its color from yellow to pink when the pH of the medium rises due to ammonia production.
Oxidase Test
Isolates were grown in BAM for 48 h at 50°C. A filter paper was placed into a petri dish and was wetted with 1% solution of tetramethyl-p-phenylenediamine. One large colony was taken with a loop and tapped lightly onto the wet filter paper. Formation of a blue-purple colour was taken as the evidence for oxidase activity.
Utilization of amino acids
Lysine and Ornithine utilization were determined by the colour change of the medium. The medium contains bromcresol purple indicator which changes its colour if the decarboxylation reaction occur under anaerobic conditions. Phenyl alanine deamination test was also done by inoculating the isolates on phenylalanine agar slants followed by addition of ferric chloride solution after incubation. The development of dark green colour with in one minute indicates a positive reaction as ferric chloride is a chelating agent and binds to phenylpyruvic acid to produce green colour.
Results and Discussion
The bacterial isolate showed a distinct zone of hydrolysis around the streak on starch-casein agar. This zone of hydrolysis confirmed the protease producing ability of the strains.
Biochemical tests for the Strain.
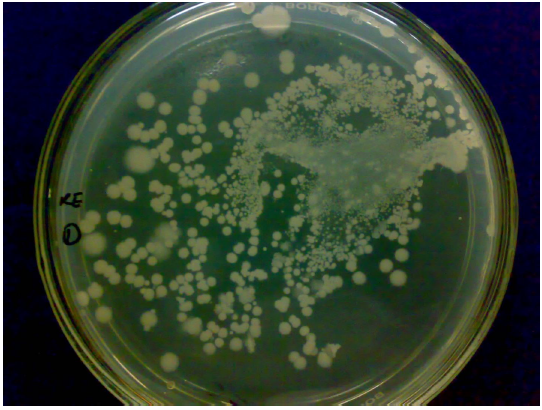
Fig. 1 Mixed plate at 10-2 serial dilution.
The mixed plate culture at 10-2 serial dilution of the kitchen exhaust samples (Fig 1).
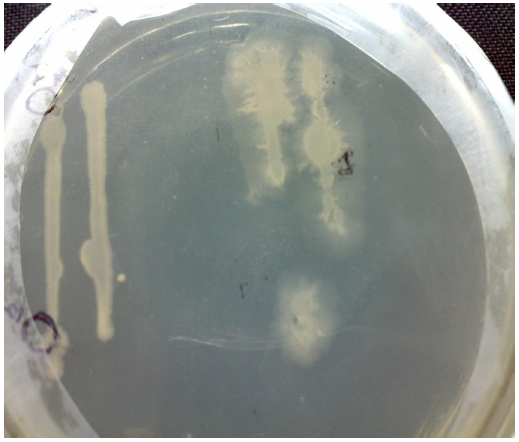
Fig. 2 Hydrolysis zone on Starch Casein agar plate
The Zone of hydrolysis of protease producing bacteria on starch casein agar is very clear and it shows this strain is having the capability of protease production (Fig. 2).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
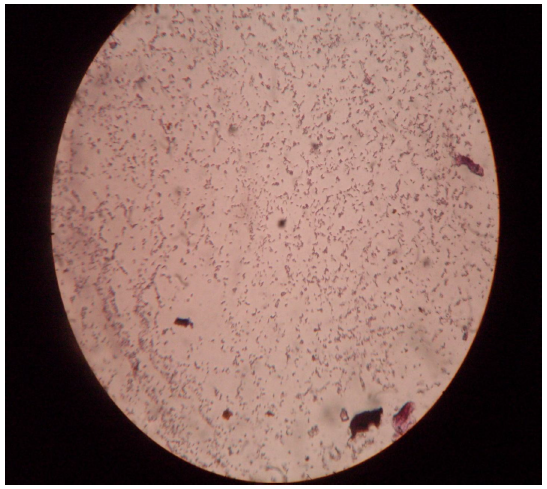
Fig. 3 Gram stain photograph
The gram negative rods of cultured strain are quite remarkable in light compound microscope ( Fig. 3).
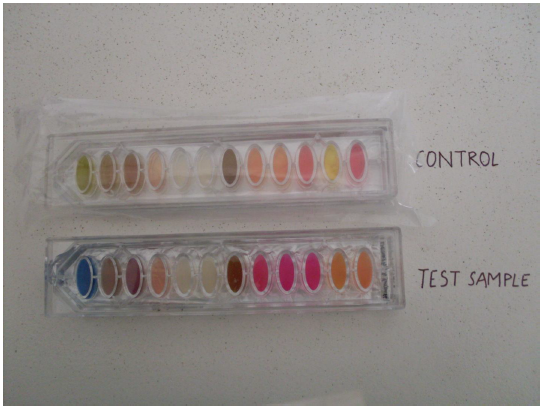
Fig. 4 Biochemical test results of the protease producing bacteria
The biochemical kit test showing change in colour from olive green to blue confirms the positive test for citrate utilization (Fig. 4).
CONCLUSION
From the above experimental work, it is concluded that kitchen exhaust contains bacterial strains, which are protease producing in nature. Thus this protease producing bacteria could be used for industrial purposes and many more.
References
Gupta R, Beg Q, Khan S and Chauhan B (2002 b) An overview on fermentation, downstream processing and properties of microbial alkaline protease. Appl. Microbiol. Biotechnol. 60: 381-395.
Gupta R, Beg Q and Lorenz P (2002 c) Bacterial alkaline proteases: molecular approaches and industrial applications. Appl. Microbiol. Biotechnol. 59: 15-32.
Gupta R, Chauhan B, Ramnani P and Singh R (2005) Bacterial alkaline proteases: Recent trends and industrial applications. In: Microbial Diversity: Current Perspectives and potential applications, Satyanarrayana T and Johri BN, eds., IK International Pvt. Ltd., New Delhi, pp 769-789.
Gupta R, Gupta K, Saxena RK and Khan S (1999) Bleach stable, alkaline protease from Bacillus sp. Biotechnol. Lett. 21: 135-138.
Christensen F (1989) Enzyme technology versus engineering technology in the food industry. Biotechnol. Appl. Biochem. 11: 249-265.
Gerhartz W (1990) Industrial use of enzymes. In: Enzymes in industry: production and application, Gerhartz W, ed., VCH Publishers, New York, pp 77-92.
Hameed A, Keshavarz T and Evans CS (1999) Effect of dissolved oxygen tension and pH on the production of extracellular protease from a new isolate of Bacillus subtilis K2, for use in leather processing. J. Chem. Technol. Biotechnol. 74: 5-8.
Singh, J.; Batra, N.; Sobti, C.R. Serine alkaline protease from a newly isolated Bacillus sp. SSR1. Proc. Biochem., 36: 781-785, 2001. Ward OP(1985). Proteolytic enzymes. In: Blanch, h.W., Drew, S., Wang, D.I., eds; comprehensive Biotechnology. Vol. 3. Oxford U.K., Pergamon Press. 789
Nunes, A.S.; Martins, M.L.L. Isolation, properties and kinetics of growth of a thermophilic Bacillus. Braz. J. Microbiol., 32: 271-275, 2001.
Pastor, M.D.; Lorda, G.S.; Balatti, A. Protease obtention using Bacillus subtilis 3411 and amaranth seed meal medium at different aeration rates. Braz. J. Microbiol., 32: 1-8, 2001.
Priest, F.G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol. Rev., 41: 711-753, 1977.
Beg ,Q.K., saxena R.K. and rani Gupta (2002) ,Kinetic constant determination for an alkaline protease from Bacillus mojavensis using RSM,
Biotechnology and Bioengineering, 78 No.3,p.289-297
Anwar, A. and Saleemuddin, M., (2000) Alkaline protease fromSpilosomaobliqua: potent applications in bioformulation. Biotechnology and applied biochemistry, vol. 31, no. 2, p. 85-89
Gupta, R; BEG, Q. K. and Lorenz, P. (2002). Bacterial alkaline proteases;molecular approaches and industrial applications. Applied microbiology and biotechnology, vol. 59, 59, no. 1, p. 15-32









