 About Authors:
About Authors:
Mr. Satyanand Tyagi*, Patel Chirag J1, Devesh Kaushik2
*President, Tyagi Pharmacy Association & Scientific Writer (Pharmacy), Chattarpur, New Delhi, India-110074.
Prof. Satyanand Tyagi is a life time member of various pharmacy professional bodies like IPA, APTI and IPGA. He has published various research papers and review articles. His academic work includes 60 Publications (50 Review Articles, 08 Research Articles and 02 Short Communications of Pharmaceutical, Medicinal and Clinical Importance, published in standard and reputed National and International Pharmacy journals; Out of 60 publications, 11 are International Publications).
He has published his papers almost in different specialization of Pharmacy field...His research topics of interest are neurodegenerative disorders, diabetes mellitus, cancer, rare genetic disorders, psycho-pharmacological agents as well as epilepsy.
1Department of Pharmaceutics, Maharishi Arvind Institute of Pharmacy, Mansarovar, Jaipur, Rajasthan, India-302020.
2Territory Business Manager, Diabetes Division, Abbott Healthcare Private Limited, Okhla, New Delhi, India- 110020.
*sntyagi9@yahoo.com, +91-9871111375/9582025220
ABSTRACT:
A group of Australian researchers at the Garvan Institute in Sydney have conducted a study indicating how Grb10 protein plays a crucial role in increasing muscle mass during development. Growth factor receptor-bound protein 10 also known as insulin receptor-binding protein Grb-IR is a protein that in humans is encoded by the GRB10 gene. The product of this gene belongs to a small family of adapter proteins that are known to interact with a number of receptor tyrosine kinases and signaling molecules. This gene encodes a growth factor receptor-binding protein that interacts with insulin and insulin-like growth-factor receptors (e.g., IGF1R and IGF2R). Over expression of some isoforms of the encoded protein inhibits tyrosine kinase activity and results in growth suppression. This gene is imprinted in a highly isoform- and tissue-specific manner. Alternatively spliced transcript variants encoding different isoforms have been identified.Scientists have discovered a way to build bigger and stronger muscles without having to lift a finger.
A study has important implications for a wide range of conditions that can worsen over time and muscle related injuries: muscular dystrophy, type 2 diabetes or problems caused by muscle inflammation. Researchers found that blocking the function of a "Hulk" protein called Grb10 in the womb produced super mice that developed bigger and stronger muscles than their normal counterparts later in life.
The aim of present article is to provide in depth knowledge about role of Hulk Protein- “Grb 10” in enhancement of muscle mass. An attempt is also made to focus on brief description of this Hulk protein so called as “Grb 10”.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1501
INTRODUCTION
Growth factor receptor-bound protein 10 also known as insulin receptor-binding protein Grb-IR is a protein that in humans is encoded by the GRB10 gene [1-3] (Fig.1-3).The product of this gene belongs to a small family of adapter proteins that are known to interact with a number of receptor tyrosine kinases and signaling molecules. This gene encodes a growth factor receptor-binding protein that interacts with insulin and insulin-like growth-factor receptors (e.g., IGF1R and IGF2R). Over expression of some isoforms of the encoded protein inhibits tyrosine kinase activity and results in growth suppression. This gene is imprinted in a highly isoform- and tissue-specific manner. Alternatively spliced transcript variants encoding different isoforms have been identified.
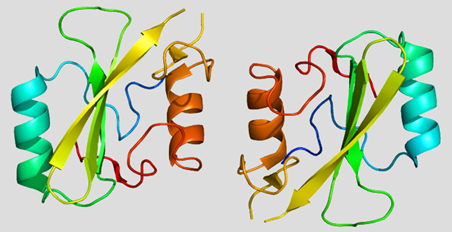
FIG. 1: Structure of the Grb10 Protein
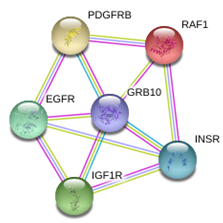
FIG. 2: Interacting Proteins for Grb10
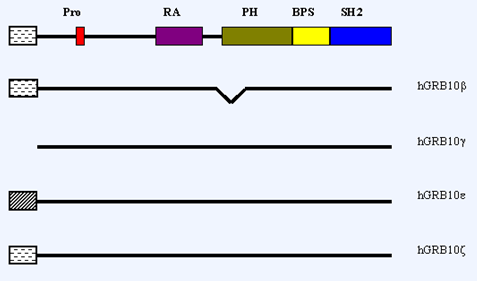
FIG. 3: GRB10 (Growth Factor Receptor-Bound Protein10)
FUNCTION, ANIMAL STUDIES AND FUNCTION
The product of this gene belongs to a small family of adapter proteins that are known to interact with a number of receptor tyrosine kinases and signaling molecules. This gene encodes a growth factor receptor-binding protein that interacts with insulin and insulin-like growth-factor receptors (e.g., IGF1R and IGF2R). Over expression of some isoforms of the encoded protein inhibits tyrosine kinase activity and results in growth suppression. This gene is imprinted in a highly isoform- and tissue-specific manner. Alternatively spliced transcript variants encoding different isoforms have been identified. Mice whose paternally inherited Grb10 gene is inactivated are more aggressive [4].GRB10 has been shown to interact with
[adsense:468x15:2204050025]
* Abl gene [5, 6]
* BCR gene [5]
* C-Raf [7, 8]
* c-Kit [9]
* Insulin receptor [6, 10, 11]
* Insulin-like growth factor 1 receptor [12, 13]
* MAP2K1 [8]
* RET proto-oncogene [14]
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
GENE SUMMARY FOR GRB10
The product of this gene belongs to a small family of adapter proteins that are known to interact with a number of receptor tyrosine kinases and signaling molecules. This gene encodes a growth factor receptor- binding protein that interacts with insulin receptors and insulin-like growth-factor receptors. Over expression of some isoforms of the encoded protein inhibits tyrosine kinase activity and results in growth suppression. This gene is imprinted in a highly isoform- and tissue-specific manner, with expression observed from the paternal allele in the brain, and from the maternal allele in the placental trophoblasts. Alternatively spliced transcript variants encoding different isoforms have been identified[15].
ASSOCIATED DISCOVERIES AND ROLE OF GRB 10 IN ENHANCEMENT OF MUSCLE MASS}
Scientists have moved closer toward helping people grow big, strong muscles without needing to hit the weight room. Australian researchers have found that by blocking the function of a protein called Grb10 while mice were in the womb, they were considerably stronger and more muscular than their normal counterparts. The discovery appears in the Journal of the Federation of American Societies for Experimental Biology (FASEB Journal) [16]. Outside of aesthetics, this study has important implications for a wide range of conditions that are worsened by, or cause muscle wasting, such as injury, muscular dystrophy, Type 2 diabetes, and problems produced by muscle inflammation.
“By identifying a novel mechanism regulating muscle development, our work has revealed potential new strategies to increase muscle mass,” said lead author Dr Lowenna Holt of the Diabetes and Obesity Research Program at the Garvan Institute of Medical Research in Sydney, Australia. “Ultimately, this might improve treatment of muscle wasting conditions, as well as metabolic disorders such as Type 2 diabetes.” To make this discovery, Dr Holt’s team compared two groups of mice. Once group had disruption of the Grb10 gene, and were very muscular. The other group, where the Grb10 gene was functional, had normal muscles. The researchers examined the properties of the muscles in both adult and newborn mice and discovered that the alterations caused by loss of Grb10 function had mainly occurred during prenatal development. These results provide insight into how Grb10, nicknamed ‘Hulk’ protein, works, suggesting that it may be possible to alter muscle growth and facilitate healing, as the processes involved in muscle regeneration and repair are similar to those for the initial formation of muscle. “Don’t turn in your gym membership just yet,” said Dr Gerald Weissmann, Editor-in-Chief of the FASEB Journal. “If you want big muscles, the classic prescription still applies: lift heavy things, eat and sleep right, and have your hormones checked. But this study shows that when we understand the basic science of how muscle fibers grow and multiply, we will be able to lift the burden – literally – of muscle disease for many of our patients [17].”
CAVEATES ASSOCIATED
Grb10 is the protein in question, and researchers at the Garvan Institute of Medical Research have found that disrupting the gene responsible for it can cause crazy boosts in muscle mass. That's right, it seems that you need to get rid of it, not get more of it. In the study, published in The FASEB Journal, a group of mice with a disrupted Grb10 gene were far more muscular than their control counterparts, both at birth and during adulthood. There are, of course, some caveats. In this study, the whole beefcake-ifying process started in the womb, so there's no hope you could get the same treatment.
These are also mice, not men. Nonetheless, this all points to an important protein that, in the future, could be extremely valuable in the treatment of people with muscle-wasting diseases, like muscular dystrophy or type-2 diabetes. It could also make you super buff, but heading to the gym is probably going to get you results a little sooner.
CONCLUSION
Scientists in Australia have discovered one of the molecular keys to a protein that promotes weight and muscle mass gain without any exercise. The tests done on mice showed that while the mice were in the womb, the functioning of Grb 10 was blocked and they were more muscular and stronger than naturally born mice. It was dubbed as the Hulk protein. This Grb 10 seems to play an important role in the promotion of muscle growth without any change in activity, diet or adverse health effects. Here, a novel mechanism has been identified that regulates the development of muscle and the work has revealed potential new strategies to increase muscle mass. The loss of Grb10 was mainly found to have happened during prenatal development thus resulting in the speculation that in the future it may be possible to alter muscle growth and heal faster, however, all this does not mean we should stop our visits to the gym; it merely opens a whole new possibility of futuristic scientific development.
ACKNOWLEDGEMENT
The corresponding author, Prof. Satyanand Tyagi is highly thankful to his Parents, Teachers, wife Pooja, and daughter Tanisha for their moral support and encouragement. Last but not the least, support of all my students and the one above all of us, the omnipresent God, for answering my prayers for giving me the strength to plod on despite my constitution wanting to give up and throw in the towel, thank you so much Dear Lord.
REFERENCES
1. Jerome CA, Scherer SW, Tsui LC, Gietz RD, Triggs-Raine B, "Assignment of growth factor receptor-bound protein 10 (GRB10) to human chromosome 7p11.2-p12", Genomics, 1997, 40 (1), 215–6.
2. Dong LQ, Du H, Porter SG, Kolakowski LF, et al, "Cloning, chromosome localization, expression, and characterization of an Src homology 2 and pleckstrin homology domain-containing insulin receptor binding protein hGrb10gamma", J. Biol. Chem, 1997, 272 (46), 29104–12.
3. Monk D, Arnaud P, Frost J, Hills FA, Stanier P, Feil R, Moore GE, "Reciprocal imprinting of human GRB10 in placental trophoblast and brain: evolutionary conservation of reversed allelic expression", Hum. Mol. Genet., 2009, 18 (16), 3066–74.
4. Garfield AS, Cowley M, et al, “Distinct physiological and behavioral functions for parental alleles of imprinted Grb10”, Nature, 2011, 469 (7331), 534-538.
5. Bai RY, Jahn T, Schrem S, Munzert G, Weidener KM, Wang JY, Duyster J, “ The SH2-containing adapter protein GRB10 interacts with BCR-ABL", Oncogene, 1998, 17 (8) : 941–8.
6. Frantz J D, Giorgetti-Peraldi S, Ottinger E A, Shoelson S E, "Human GRB-IRbeta/GRB10. Splice variants of an insulin and growth factor receptor-binding protein with PH and SH2 domains", J. Biol. Chem, 1997, 272 (5): 2659–67.
7. Nantel A, Hubber M, Thomas DY, Localization of endogenous Grb10 to the mitochondria and its Interaction with the mitochondrial- associated Raf-1 pool", J. Biol. Chem, 1999, 274 (50), 35719–24.
8. Nantel A, Mohammad-Ali K, Sherk J, Posner B I, Thomas D Y, "Interaction of the Grb10 adapter protein with the Raf1 and MEK1 kinases", J. Biol. Chem, 1998, 273 (17), 10475–84.
9. Thomas Ahn, Petra Seipel, Urschel Susanne, Peschel Christian, Duyster Justus, “Role for the adaptor protein Grb10 in the activation of Akt”, Mol. Cell. Biol, 2002, 22(4), 979-91.
10. Langlais P, Dong L Q, Hu D, Liu F, "Identification of Grb10 as a direct substrate for members of the Src tyrosine kinase family", Oncogene, 2000, 19 (25), 2895–903.
11. Hansen H, Svensson U, Zhu J, Laviola L, Giorgino F, Wolf G, Smith R J, Riedel H, "Interaction between the Grb10 SH2 domain and the insulin receptor carboxyl terminus", J. Biol. Chem, 1996, 271 (15), 8882–6.
12. He W, Rose D W, Olefsky J M, Gustafson T A, "Grb10 interacts differentially with the insulin receptor, insulin-like growth factor I receptor and epidermal growth factor receptor via the Grb10 Src homology 2 (SH2) domain insulin-like growth factor ", J. Biol. Chem, 1998, 273 (12), 6860–7.
13. Vecchione Andrea, Marchese Adriano, Henry Pauline, Rotin Daniela, Morrione Andrea, "The Grb10/Nedd4 complex regulates ligand-induced ubiquitination and stability of the insulin-like growth factor I receptor", Mol. Cell. Biol, 2003, 23 (9), 3363–72.
14. Pandey A, Duan H, Di Fiore PP, Dixit VM, “The Ret receptor protein tyrosine kinase associates with the SH2-containing adapter protein Grb10”, J.Biol.Chem, 1995, 270(37), 21461-3.
15. genecards.org/cgi-bin/carddisp.pl?gene=GRB10
16. sci-news.com/medicine/article00560.html
17. Lowenna J. Holt, et al, Grb10 regulates the development of fiber number in skeletal muscle, FASEB J, 2012, 26, 3658-3669.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









