{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Devender M. Sharma*1, Satish B. Kosalge1, Swati N. Lade1
Hi – Tech college of pharmacy, Chandrapur (M.S.)
*sdevender350@gmail.com
ABSTRACT
Tablets are unit solid dosage form of medicament which largely used to compare other dosage form of medicaments. In tablet manufacturing, wet granulation and dry granulation methods largely used as compare to MADG process. In wet granulation and dry granulation methods various steps has been carried out like dispensing and shifting, dry mixing, granulation, pre-drying, shifting, premixing, lubrication and compression. But in MADG process, escape various steps like slugging, pre- drying, shifting, and drying. Hereby in MADG process save energy, cost of product and time also. The main object of review is hereby moisture activated dry granulation process very beneficial in case of water insoluble drug and water poorly soluble drug other than granulation method like wet granulation. Another objective of review how moisture activated dry granulation process carried out and given various advantages over the other granulation method. In water insoluble drug case MADG process largely used and very useful method for pharmaceutical industries.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2552
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 5, Issue 12 Received On: 21/09/2017; Accepted On: 23/09/2017; Published On: 01/12/2017 How to cite this article: Sharma DM, Kosalge SB, Lade SN;Review on Moisture activated Dry Granulation Process; PharmaTutor; 2017; 5(12); 58-67 |
INTRODUCTION
Tablet is a unit solid dosage form containing active ingredient with or without suitable excipients. These are most widely used dosage form. The main objective of the design and manufacture of the compressed tablet is to deliver orally correct amount of drug in the proper form over proper time and at desired location, so as to have suitable chemical integrity protected at the point of its action. (Aniket et al., 2011)The physical design, manufacturing process, and complete chemical makeup of the tablet can have a profound effect on the efficiency of the drug being administered. (Aniruddha et al., 2001) Poorly water soluble drugs are associated with slow drug absorption leading eventually to inadequate and variable bioavailability and nearly 40% of the new chemical entities currently being discovered are poorly water-soluble drugs. (B.Venkateswara et al., 2014) Based upon their permeability characteristics, the Biopharmaceutics classification system (BCS) classifies such drugs in two major classes, i.e., Class II and IV. The BCS class II drugs are poorly water-soluble entities with high permeability. Most formulation strategies for such drugs are targeted at enhancing their fine dispersion at absorption level. (Devender et al., 2017) Ibuprofen being poorly water-soluble drug known to demonstrate dissolution or solubility limited absorption. The bioavailability of the drug is low, yet its rate of absorption is quite inconsistent and delayed with time. Based upon its aqueous solubility and various dissolution parameters, the drug bioavailability can unambiguously be regarded as limited solely to dissolution. (Devender et al., 2017)
Tablet Manufacturing Process
Tablet manufacturing process can be broadly classified as granulation and direct compression. Granulation process may be defined as the size enlargement process in which fine or coarse particles is converted into physically stronger and larger agglomerates having good flow properties, better compression characteristics and uniformity and a process for collecting particles together by creating bonds between them. It is the most widely used technique in the pharmaceutical industry for the preparation of materials for tabletting. (Sharma et al., 2007)
Granulation may be either wet granulation or dry granulation i.e., by using binder solution or, by using dry binder. Pharmaceutical granules typically have a size range between 0.2 to 4.0 mm, depending on their subsequent use. Most of formulation in tablet manufacturing is by wet granulation process. (Devender et al., 2017)
Granulation
Granulation is the process in which primary powder particles are made to adhere to form larger, multi particle aggregates called granules. After granulation process the granules may either be packed (when used as a dosage form - powder), or they may be mixed with other excipients prior to tablet compaction or capsule filling. (Chen et al., 1990) Granulation is used mainly to improve flow and compressibility of powders so as to prevent segregation of the blend components to improve content uniformity, and eliminate excessive amounts of fines. Particle size of the granulate is mainly affected by the quantity and feeding rate. (Christensen et al., 2009).
Granulation method can be broadly classified into two types: (Devender et al., 2017)
A) Wet granulation
B) Dry granulation
C) Direct compression
Wet granulation
A. Steps involved in the wet granulation
a. Mixing of the drugs and excipients
b. Preparation of binder solution
c. Mixing of binder solution with powder mixture to form wet mass.
d. Coarse screening of wet mass using a suitable sieve (6-12 # mesh)
e. Drying of moist granules.
f. Screening of dry granules through a suitable sieve (14-20 # mesh)
g. Mixing of screened granules with disintegrates, glidant, and lubricant.
B. Special wet granulation techniques
a. High shear mixture granulation
b. Fluid bed granulation
c. Extrusion-spheronization
d. Spray drying Dry granulation
The process mainly involves a series of operations such as weighing, mixing, granulation, screening the damp mass, drying, dry screening, lubrication and finally compression. However, as all of these operations are time bound, problem of reproducing uniform granulation from one lot to the next, is the greatest disadvantage of this method. (Devender et al., 2017) The active ingredients, diluents and disintegrants are mixed and blended well. Twin-shell blender or Ribbon blender may be employed for large scale blending. The blended material is then shifted through a screen of suitable mesh to remove or break the lumps. The solution of binding agent is added to the powder with stirring till the mass attains the consistency of damp snow or brown sugar. A Sigma blade mixer or Twin-shell blender may be used in pharmaceutical industry for this purpose. The damp mass is then force through a six or eight mesh screen. A manual screen is used for small batches but for larger quantities one of several comminuting mills suitable for wet screening can be used. A Fitzpatrick comminuting mill is the equipment of choice. The granular material thus obtained is dried on trays in a hot air oven or in a fluid bed dryer. (Deepak et al., 2017) Oven drying may take about 12hours or longer. During drying the particles may agglomerate and form lumps and therefore a dry screening operation is usually required after drying. (Himanshu et al., 2010) An oscillating granulator is often suited for the purpose. This is followed by the addition of lubricant as a fine powder (usually 100 # mesh). Excessive amount of fines or the fine powders should be avoided however10-20% fines facilitate the preparation of tablets of uniform weight. (Dong et al., 2008)
Tablet granulation can also be prepared rapidly by utilizing the air suspension coating technique. In this method, particles of active drug or inert material are suspended in the vertical column and the solution of granulating material is sprayed. The particle size gradually increase and the granules are obtained. (Devender et al., 2017)
When a soluble die is incorporated in the granulating agent, wet granulation method can produce uniformly tablets. Wet granulation is best suited to tablet making for potent drugs given in the minute doses. Wet granulation technique is receiving great significance, because direct compression method is not most suitable technique for many active substances that are in high dosages or in fine powder form. (Nidhi et al., 2014)
Granulation is one of the most common unit operation employed to improve the flow and compressibility of particulate material by size enlargement and densification. Granulation can be divided into wet and dry method. (Nidhi et al., 2014) Wet granulation method is more often chosen over dry granulation because of dust elimination, single pot processing and obtaining predictable granulation end point determination. Example of wet granulation method include fluid bed, high shear, pelletization techniques such as extrusion-spheronization, spray drying, rotary processing and so forth. In wet granulation, a liquid is use to agglomerate powder particles with constant agitation into a coherent mass which is subsequently dried and sized for subsequent processing. Despite their advantages, wet granulation method is not suitable for thermo-labile and moisture sensitive materials or materials that are highly cohesive. (Puckhraj et al., 2012)
Most drug actives do not possess adequate flow properties and compressibility to produce a final dosage form of desired physical and mechanical properties. (Ranpise et al., 2013) These properties include content uniformity, low friability, strong enough for subsequent handling and processing such as coating and packaging and good dissolution profiles to achieve the requisite bioavailability. (Hong-Liang et al., 2008) Hence, other material or excipients are often added to the powdered active to improve the tablet ability of the overall blend. Depending on the nature of the actives, excipients such as MCC pH 101, MCC pH 102, spray dried lactose and starch 1500 can be added to provide a balance of plasticity and brittle fracture needed to bring about successful bonding within a tablet. (Ismat et al., 2009)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Advantages
a) Permits mechanical handling of powders without loss of quality of blend.
b) The flow properties of powder are improved by increasing particle size and sphericity.
c) Increases and improves the uniformity of powder density.
d) Improves cohesion during and after compaction.
e) Air entrapment is reduced.
f) Reduces the level of dust and cross contamination.
g) Allows for the addition of a liquid phase to powders.
h) The hydrophobic surfaces are made hydrophilic.
Limitation of wet granulation
a. The greatest disadvantage of wet granulation is its cost. It is an expensive process because of labor, time, equipment, energy and space requirements.
b. Loss of material during various stages of processing.
c. Stability may be major concern for moisture sensitive or thermo labile drugs.
d. Multiple processing steps add complexity and make validation and control difficult.
e. An inherent limitation of wet granulation is that any incompatibility between formulation components is aggravated. (Lachman, 1990)
Dry granulation
Dry granulation is also known as ‘slugging’, double compression or recompression method. (Khinchi et al., 2012) It can be used when the tablet ingredients are sensitive to moisture or are unable to withstand elevated temperature during drying. Under these circumstances, dry granulation is the method of choice provided the tablet ingredients have sufficient inherent binding or cohesive properties. (Nair et al., 2010) Essential steps in this method include weighing, mixing, dry blending, dry screening, lubrication and compression. (Remington, 2006)
Advantages:
The main advantages of dry granulation
a. For moisture sensitive material
b. For heat sensitive material
c. For improved disintegration since powder particles are not bonded together by a binder.
Disadvantages:
a. It requires a specialized heavy duty tablet press to form slug.
b. It does not permit uniform colour distribution as can be achieved with wet granulation where the dye can be incorporated into binder liquid.
c. The process tends to create more dust than wet granulation, increasing the potential contamination. (Devender et al., 2017)
Direct Compression
Direct compression is the tabletting of a blend of ingredients, the compression mix, without a preliminary granulation or aggregation process. (Sheskey et al., 2003) The compression mix contains the active pharmaceutical ingredients (API) blended with one or more excipients. The excipients may include binders, filler/diluents, disintegrates, and lubricants such DC compression mixes must flow uniformly into a die and form a robust tablet. (Thejaswini et al., 2013)
Before the 1960s, most tablet production required granulation of the powdered constituents prior to tabletting. The primary purpose of granulation is to produce a free flowing compression mix with acceptable compactability. (Railmkar et al., 2000) The availability of DC grade excipients, and faster tablet machines with assisted feed and precompression, enabled the rise of DC. The first significant discussion of the concept of DC was presented by Milosovitch in 1962.
The distinction between DC and wet or dry granulation is not absolute, as the addition of extragranular ingredients (“post-granulation running powder”) constitutes a DC step, where the granulate itself can be regarded as one of the DC ingredient. As granulation does not always deliver the necessary compactability the use of microcrystalline cellulose (MCC) post granulation to increase tablet hardness has been common practice since the introduction of DC. Blending and compaction are two unit processes common to both wet/dry granulation and DC. (Sarath et al., 2011)
Advantages of Direct Compression:
a. Cost Effectiveness
b. Stability
c. Faster Dissolution
d. Less wears & tears of punches
e. Simplified Validation
Limitations of direct compression
a. Segregation
b. Low dilution potential
c. Re-workability
d. Lubricant sensitivity
e. Variation in functionality
Ideal characteristics of granules
a. Uniformity
b. Spherical shape
c. Smaller particle size distribution with sufficient fines to fill void spaces between granules, adequate moisture (between 1-2%)
d. Good flow
e. Good compressibility and
f. Sufficient hardness
The effectiveness of granulation depends on the following properties
a. Particle size of the drug and excipients
b. Type of binder (strong or weak)
c. Volume of binder (less or more)
d. Wet massing time (less or more)
e. Amount of shear applied
f. Drying rate (Hydrate formation and polymorphism)
Physiological properties
a. bulk density
b. tapped density
c. hausners ratio
d. compressibility index
|
DRY GRANULATION |
WET GRANULATION |
MOISTURE ACTIVATED DRY GRANULATION |
|
|---|---|---|---|
|
Dispensing and Shifting |
Dispensing and Shifting |
Dispensing and Shifting |
|
|
Dry mixing |
Dry mixing
|
Dry mixing |
|
|
Slugging |
|
Granulation
|
Granulation |
|
Half lubrication |
Lubrication |
Pre-drying |
|
|
Compression |
Compression |
Shifting |
|
|
Milling |
|
Drying |
|
|
Shifting |
|
Pre-mixing (unlubrication) |
Pre-mixing (unlubrication) |
|
Final lubrication |
|
Lubrication |
Lubrication |
|
compression |
|
Compression |
Compression |
Figure 1: Compare between dry granulation, wet granulation and moisture activated dry granulation
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Advanced Granulation Techniques Over a period of time, due to technological advancements and in an urge to improve commercial output various newer granulation technologies have been evolved such as
a. Steam Granulation (SG)
b. Moisture Activated Dry Granulation (MADG)
c. Pneumatic Dry Granulation (PDG)
d. Freeze granulation Technology (FGT)
e. Foamed Binder Technologies (FBT)
f. Melt Granulation Technology (MGT)
g. Thermal Adhesion Granulation Process (TAGP)
h. Granulex Technology (GT)
i. Foam Granulation (FG)
Steam Granulation (SG)
It is modification of wet granulation. Here steam is used as a binder instead of water. In this method of granulating particles involves the injection of the required amount of liquid in the form of steam. This steam injection method, which employs steam at a temperature of about 150°C which tends to produce local overheating and excessive wetting of the particles in the vicinity of the steam nozzles, thereby causing the formation of lumps in the granulated product.
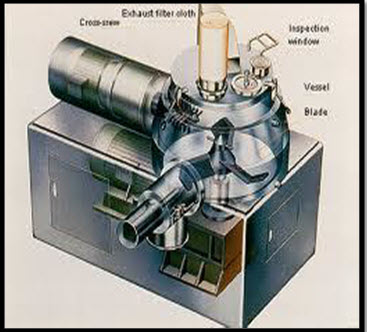
Figure 2: Steam granulation machine
Advantage:
a. Higher distribution uniformity.
b. Higher diffusion rate into powders.
c. Steam granules are more spherical
d. Have large surface area hence increased dissolution rate of the drug from granules.
e. Processing time is shorter therefore more number of tablets is produced per batch.
Pneumatic Dry Granulation (PDG)
It is a novel dry method for automatic and semiautomatic production of granules.PDG Technology has been used with superior results in developing fast-release, controlled-release, fixed-dose, and orally disintegrating tablets. The technology is applicable to practically any solid dosage pharmaceutical product. (Zhaib et al., 2009)
PDG is an innovative form of dry granulation with the same process up to Roller Compaction
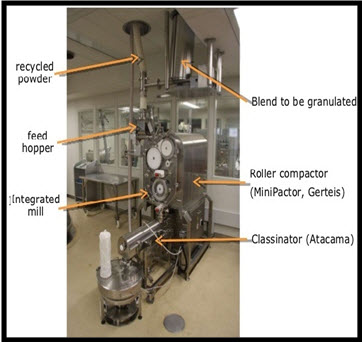
Figure 3: Pneumatic Dry Granulation
Advantages of PDG Technology
a. Faster speed of manufacturing compared with wet granulation.
b. Lower cost of manufacturing compared with wet Granulation.
c. The end products are very stable - shelf life may be enhanced.
d. Little or no wastage of material.
e. Scale-up is straightforward.
Freeze granulation Technology

Figure 4: Freeze granulation
Freeze granulation (FG) – which enables preservation of the homogeneity from suspension to dry granules by spraying a powder suspension on liquid nitrogen, the drops (granules) was instantaneously frozen. In a subsequent freeze-drying the granules are dried by sublimation of the ice without any segregation effects as in the case of conventional drying in air. The result will be spherical, free flowing granules, with optimal
homogeneity. Crushing to homogeneous and dense powder compacts in a pressing operation. High degree of compact homogeneity will then support the following sintering with minimal risks for granule defects.
Advantages:
a. Mild drying prevents serious oxidation of non-oxides and metals.
b. No cavities in the granules.
c. Low material waste (high yield).
d. Easy clean of the equipment (latex binder can be used).
Foamed Binder Technologies (FBT)
These technology using proven for METHOCEL polymers, this technology can greatly improve binder distribution in the formulation mix and yields a remarkable array of processing. Compared to conventional spray processing, foamed binder technology can shorten processing times by reducing water requirements. It can improve reproducibility through more uniform binder distribution.

Figure 5: Foamed Binder Technologies
Moreover, it eliminates spray nozzles and their many variables in granulation processing equipment. Foam processing also offers better end point determinations and reduced equipment clean-up time. While foamed binder processing offers many advantages, this technology doesn’t demand new equipment or radical changes in processing techniques.
Melt Granulation Technology
Melt granulation is processes by which granules are obtained through the addition of either a molten binder or a solid binder which melts during the process. This process is also called melt agglomeration and thermoplastic granulation.

Figure 6 : Melt Granulation Technology
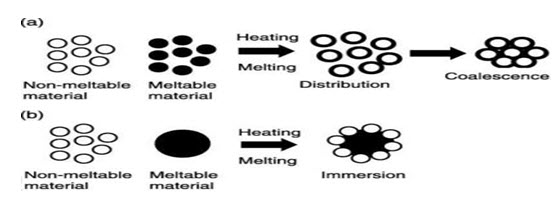
Figure 7: Principle of melt granulation
Advantage of melt granulation:
a. Neither solvent nor water used.
b. Fewer processing steps needed thus time consuming drying steps eliminated.
c. Uniform dispersion of fine particle occurs.
d. Good stability at varying pH and moisture levels.
e. Safe application in humans due to their non-swellable and water insoluble nature.
Thermal Adhesion Granulation Process (TAGP)
It is applicable for preparing direct tabletting formulations. TAGP is performed under low moisture content or low content of pharmaceutically acceptable solvent by subjecting a mixture containing one or more diluents and/or active ingredients; a binder; and optionally a disintegrant to heating at a temperature in the range from about 30ºC to about 130ºC in a closed system under mixing by tumble rotation until the formation of granules. This method utilizes less water or solvent than traditional wet granulation method it provides granules with good flow properties and binding capacity to form tablets of low friability, adequate hardness and have a high uptake capacity for active substances whose tabletting is poor.
a. In thermal adhesion granulation, granules formed during mixing of moist powder under continuous tumble rotation, as the heated powder mass flows within the container and agglomerates with the aid of the binder drying and milling to form the desired granules are unnecessary in the present invention due to the low amount of moisture introduced to the tabletting mixture.
Advantages of Thermal Adhesion Granulation Process:
a. It helps to minimize the generation of dust during powder processing.
b. This technique serves to contain fine-powder active ingredients whose spread or loss from the system is not desirable due to their cost or biological activity.
Moisture Activated Dry Granulation (MADG)
Moisture Activated Dry Granulation (MADG) was developed in response to the difficulties experienced with wet granulation, in terms of endpoint, drying and milling. Wet granulation process endpoint is very sensitive to granulation time and shear. The wet granules need to be dried to a narrow range of moisture contents, which is difficult. The dried granules need to be milled, but the milled granules often have either too many fines or too many coarse particles (or both) an undesirable bimodal distribution.
MADG is a very simple and innovative process where granules are created with water and a granulating binder, as in wet granulation, but are not heat dried or milled. This process helps to minimise endpoint sensitivity.
MADG is a very simple and innovative process where granules are created with water and a granulating binder, as in wet granulation, but are not heat dried or milled. This process helps to minimize endpoint sensitivity.
It is applicable to many of the pharmaceutical industry's granulation needs for solid dosage form development and can be described as a 'one-pot' granulation process.
Moisture Activated Dry Granulation (MADG) was developed in response to the difficulties experienced with wet granulation, in terms of endpoint, drying and milling. Wet granulation process endpoint is very sensitive to granulation time and shear. The wet granules need to be dried to a narrow range of moisture contents, which is difficult. The dried granules need to be milled, but the milled granules often have either too many fines or too many coarse particles (or both) an undesirable bimodal distribution.
The MADG process
MADG has two stages: agglomeration and moisture distribution. Success depends on the selection and order in which the formulation ingredients are added, as well as how the process is carried out.
During agglomeration, a major portion of the formulation containing the drug is agglomerated. The drug is blended with filler and binder in the powder form, and this blend constitutes approximately 50–80% of the formula weight. In the second stage, a small amount (0%, 0.5%, 1%, 1.5%, 2%, 2.5%, 3%, 3.5%, 4%, 4.5%, and 5%) of water is sprayed as small droplets onto the blend (while blending). (Devender et al., 2017)Water moistens the blend and causes the binder to become tacky, which causes particles, particularly fines, to form moist agglomerates. The process does not create large granules, which would need milling, and because very little water is used in the process, the endpoint is not sensitive to blending.

Figure 8: Moisture activated dry granulation
In this method moisture is used to activate the granule formation but the granules drying step is not necessary due to moisture absorbing material such as MCC, aerosol, maize starch. The moisture-activated dry granulation process consists of two steps, wet agglomeration of the powder mixture followed by moisture absorption stages. A small amount of water (0%, 0.5%, 1%, 1.5%, 2%, 2.5%, 3%, 3.5%, 4%, 4.5%, and 5%) is added first to agglomerate the mixture of the API, a binder, and excipients. Moisture absorbing material such as MCC, aerosil and maize starch is then added to absorb any excessive moisture. (Zhaib et al., 2009) After mixing with a lubricant, the resulting mixture can then be compressed directly into tablets. Hence, this process offers the advantage of wet granulation is that eliminates the need for a drying step. (Devender et al., 2017)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Moisture Activated Dry Granulation (MADG) is a process in which moisture is used to activate granule formation, without the need to apply heat to dry the granules. There are two main stages in MADG: Agglomeration Moisture distribution/ Absorption During agglomeration, drug is mix with diluents and binder in the granulator, to obtain a uniform mixture. This blend constitutes approximately 50- 80% of formula weight. (Devender et al., 2017) While mixing, a small amount of water (0%, 0.5%, 1%, 1.5%, 2%, 2.5%, 3%, 3.5%, 4%, 4.5%, and 5%) is sprayed as small droplets onto the powder blend, which moistens the binder and makes it tacky. The particle size of the agglomerates generally falls in the range of 150–500 µm.
Advantages
a. Short processing time.
b. Suitable for continuous processing.
c. It uses very little energy.
d. In essence, MADG is just a creative form of wet granulation: granules are created with water with the help of granulating material, but no more water is added than necessary.
e. It utilizes very little granulating fluid.
f. It decreases drying time and produces granules with excellent flow ability.
g. Single production equipment (high shear granulator)
h. No equipment change
i. Lower tablet capping
j. No over and under granulation
k. Time and cost effective, as it eliminates the liquid addition and drying steps.
l. Reproducible and scalable
m. Applicable to a number of soluble and insoluble drug formulations
Disadvantages
a. Heat sensitive materials are poor candidates.
b. Moisture sensitive and high moisture absorbing API is poor candidates.
c. Formulations with high drug loading are difficult to develop.
d. Could be other issues with the API, with high-drug load formulations being particularly difficult to develop.
e. Less familiarity with the process. (Devender et al., 2017)
CONCLUSION
The moisture activated dry granulation method was found to be simple, reproducible, easily controllable, economical, and continues process. Additionally, the excipients used for the formulation of tablets were cheap and easily available. Other drugs for the use in moisture activated dry granulation method can be incorporated in the formulation of tablets. Therefore, these types of moisture activated dry granulation method for tablets can be commercially processed easily and potentially better other than wet granulation method for formulation of tablets.
REFERENCES
1. Ankit Sharma, Pooja sethi, Dinesh pawar. “Granulation techniques and innovations”, Inventi Rapid: Pharmtech, Vol.10, 2011.
2. Aniruddha MR, Joseph BS. Use of a moist granulation technique to develop controlled-release dosage forms of acetaminophen. Drug Dev Ind Pharm 4 ; 27,337 -43, 2001.
3. B.Venkateswara Reddy, K. Navaneetha, P. Sandeep. Improved tablet production by modified granulation techniques, International Journal of research in pharmacy and life sciences, 2(2), 224-235, 2014.
4. Chen CM, Dhananjaya A, Michael RI, Jeffrey LC. Comparison of moisture-activated dry granulation processes with conventional granulation methods for sematilide hydrochloride tablets. Drug DevInd Pharm, 16(3), 379-941990.
5. Christensen LH, Johansen HE, Schaefer T. Moisture-activated dry granulation in a high shear mixer. Drug Dev Ind. Pharm, 20(14), 2195-2213, 2009.
6. Devender Sharma, Ameya lanjewar, M.D.Godbole and Susil Burle research article on “Formulation and evaluation on water poorly soluble drug tablets by madg method” , WJPR; 2017.
7. Deepak Kashyap, S.L.Kokhra, Shalini Sharma, “Development of enteric coated tablets of ibuprofen using methacrylic acid copolymer, international journal of institutional pharmacy and life science, 2(1):2249-6807,2017.
8. D. K. Sharma and S.B. Joshi, Solubility enhancement strategies for poorly water-soluble drugs in solid dispersions: A Review, Asian Journal of Pharmaceutics Volume 1, Issue 1, April - June, 2007.
9. Dong Xun Li, Yu-Kyuong Oh, Soo-Jeong Lim, Jong Oh Kim, Ho Joon Yang, Jung Hoon Sung, Chul Soon Yong, Novel gelatin microcapsule with bioavailability enhancement of ibuprofen using spray-drying technique, International Journal of Pharmaceutics Volume 355, Issues 1-2, 277-284, 2008.
10. Himanshu.K.Solanki, Tarashankar Basuri, Jalaram H.Thakkar, “Recent advances in granulation technology, International Journal of Pharmaceutical Sciences Review and Research, 3(5), 48,2010 .
11. Nidhi Prakash Sapkal, Vaishali A. Kilor, Minal Nandkumar Bonde. Application of a convenient and cost and effective granulation technology for the formulation of tablets using conventional excipients, Asian Journal of Pharmaceutics - 225-254, 2014.
12. Puckhraj Chhaprel, Amit Talesara, Amit K Jain. Solubility enhancement of poorly water soluble drug using spray drying technique,” International Journal of Pharmaceutical Studies and Research, Vol. III, Issue II, April-June,.01-05, 2012.
13. N. S. Ranpise and a. A. Borse. Review on novel granulation techniques, journal of pharmaceutical associations India, Ranpise et al., JPAI, , 1(1), 1-13, 2013.
14. Hong-Liang L, Hsiu-O H, Chi-Chia C, Ta-Shuong Y. Process and formulation characterizations of the thermal adhesion granulation (TAG) process for improving granular properties, International J Pharm, 357(1-2):206-212,2008.
15. Ismat U, Jennifer W, Shih-YC, Gary JW, Nemichand BJ, San K. Moisture activate dry granulation-Part I: A guide to excipient and equipment selection and formulation development, Pharm Tech, 33(11), 62-70, 2009.
16. Lachman L, Lieberman HA, Joseph LK. The Theory and Practice of Industrial Pharmacy,;Third Edition, 317-324,1990.
17. M. P. khinchi, dilip Agrawal , M.K. Gupta, “Recent advancement in tablet technology, international journal of pharmaceutical research and development, 1-30, 2012.
18. Nair AB, Gupta R, Kumria, Formulation and evaluation of enteric coated tablets of proton pump inhibitor. J Basic clinical pharma; 1 (4): 215-221, 2010.
19. Remington J. Remington: The Science and Practice of Pharmacy; twenty first edition, Lippincott Williams & Wilkins, 895-899, 2006.
20. Sheskey P. et al., "Foam Technology: The Development of a Novel Technique for the Delivery of Aqueous Binder Systems in High-Shear and Fluid- Bed Wet-Granulation Applications," poster presented at AAPS Annual Meeting and Exposition, Salt Lake City, UT, 26-30, 2003.
21. P. Thejaswini, B. Suguna, N. Sumalatha, Advance granulation techniques for pharamceutical pharmulations, International journal of research in pharmaceutical and nano sciences, 2(6), 723-732,2013.
22. Railmkar A., et al, Evaluation and Comparison of a Moist Granulation Technique to Conventional methods. Drug Dev Ind. Pharm., 26(8), 855-889, 2000.
23. Sarath S. Menon, B. V. Basavaraj, S. Bharat, “Formulation and evaluation of ibuprofen tablets using orange peel pectin as binding agent. Scholars research library, 3(4), 241-247, 2011.
24. Zhaib H, Lia S, Andrewsb G, Jonesb D, Bella S, Walkera G. Nucleation and growth in fluidised hot melt granulation and Powder Technology.;189(2), 230-237, 2009.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









