{ DOWNLOAD AS PDF }
 ABOUT AUTHORS:
ABOUT AUTHORS:
Asif I Bhim*, Vineet Jain, Hasumati Raj
Shree Dhanvantary Pharmacy College,
Kim, Gujarat, India
bhimiqbal23@gmail.com
ABSTRACT:
Itopride Hydrochloride is a novel, synthesized, gastro prokinetic drug, which stimulates gastrointestinal motor activity through the synergistic effects of dopamine D2-receptor blockade and acetylcholine esterase inhibitors. Chemically, it is N-[[4-[2-(Dimethyl amino) ethoxy] phenyl] methyl]-3, 4-dimethoxy benzamide hydrochloride. Benzamide structure, amide and ether linkages in the drug molecule make it susceptible to degradation. Thus a prokinetic drug like Itopride Hydrochloride by virtue of its efficacy and tolerability could be considered as a drug of first choice and a welcome addition to the drug armamentarium for the symptomatic treatment of NUD (non-ulcer Dyspepsia) and other gastric motility disorders including functional bowel disorders. This review consists of various analytical methods for determination of Itopride Hydrochloride in various marketed pharmaceutical preparation and in biological fluids. Analytical method consists of various spectroscopic methods, chromatographic methods and other methods.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2261
|
PharmaTutor (ISSN: 2347 - 7881) Volume 2, Issue 10 How to cite this article: AI Bhim, V Jain, R Hasumati; A Review: Determination of Itopride Hydrochloride in biological fluid and Pharmaceutical Dosage Forms; PharmaTutor; 2014; 2(10); 38-44 |
INTRODUCTION:[1-6]
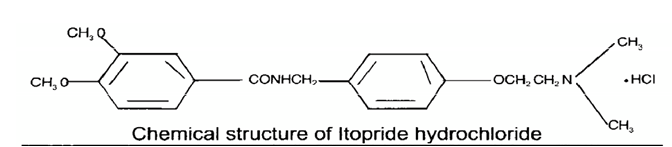
STRUCTURAL FORMULA: [4]
MOLECULAR FORMULA: C20H26N2O4HCL
MOLECULAR WEIGHT: 394.93 g/mol
CHEMICAL NAME:
N-[[4-[2-(Dimethyl amino) ethoxy] phenyl] methyl]-3, 4-dimethoxy benzamide hydrochloride.
CATEGORY: Anticholinesterase
DOSE: 150 mg daily
DESCRIPTION: white amorphous powder
SOLUBILITY:
Soluble in methanol, water, dimethyl sulphoxide, N, N-dimethyl formemide. Springily soluble in ethanol, propylene glycol, polyethylene glycol. Very slightly soluble in hexane, dichloromethane, methyl benzene.
[adsense:468x15:2204050025]
PHARMACOLOGICAL ACTION:
Itopride has anticholinesterase (AchE) activity as well as dopamine D2 receptor antagonistic activity and is being used for the symptomatic treatment of various gastrointestinal motility disorders. It is well established that M3 receptors exist on the smooth muscle layer throughout the gut and acetylcholine (ACh) released from enteric nerve endings stimulates the contraction of smooth muscle through M3 receptors. The enzyme AChE hydrolyses the released ACh, inactivates it and thus inhibits the gastric motility leading to various digestive disorders. Besides ACh, dopamine is present in significant amounts in the gastrointestinal tract and has several inhibitory effects on gastrointestinal motility, including reduction of lower esophageal sphincter and intragastric pressure. These effects appear to result from suppression of ACh release from the myenteric motor neurons and are mediated by the D2 subtype of dopamine receptors. Itopride, by virtue of its dopamine D2 receptor antagonism, removes the inhibitory effects on Ach release. It also inhibits the enzyme AchE which prevents the degradation of Ach.
The net effect is an increase in ACh concentration, which in turn, promotes gastric motility, increases the lower esophageal sphincter pressure, accelerates gastric emptying and improves gastro-duodenal coordination (Figure)
This dual mode of action of Itopride is unique and different from the actions of other prokinetic agents available in the market.
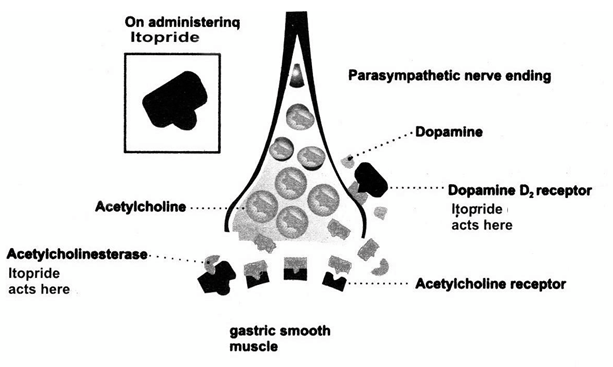
Figure: Mode of action of Itopride. [4]
PHARMACOKINETICS:
On oral administration, Itopride is rapidly and extensively absorbed and peak serum concentrations are achieved within 35 minutes after oral dosing. Thus it has a rapid onset of action, unlike cisapride and mosapride, which take around 60 minutes to reach peak plasma concentrations. Itopride is metabolized in the liver by N-oxidation to inactive metabolites by the enzyme flavin-containing monooxygenase (FMO).The half-life of Itopride is about 6 hours. It is excreted mainly by the kidneys as metabolites and unchanged drug
SIDE EFFECTS:
Rash, diarrhea, giddiness, exhaustion, back and chest pain, increased salivation, constipation, abdominal pain, headache, sleeping disorder, dizziness, galactorrhea and gynecomastia.
ANALYTICAL METHODS:
This all are the methods which are used for the determination of Itopride Hydrochloride in marketed formulation and in biological fluids. This all analytical methods are reported which are seen during the literature survey. This article describes the review on the all reported analytical methods with specific conditions.
I. COMPENDIAL METHODS:
Itopride Hydrochloride is not official in any pharmacopeia.
II. CHROMATOGRAPHIC METHODS: [7-14]
Various chromatographic methods are used for the determination of the Itopride Hydrochloride alone or combination with other drugs in various marketed formulation and in biological fluids like human plasma and urine. Chromatographic methods like High performance liquid chromatography (HPLC/RP-HPLC), High performance thin layer chromatography (HPTLC) with UV detection or with flourometric detection are used. In which the stationary phase commonly used is C18 column and commonly used wavelength for detection is 258nm.Mobile phase is varies with condition of method in various proportion. Below in table describes the summary of the various chromatographic methods are used with the method description.
Table No.1: Summary of Chromatographic Methods of Itopride hydrochloride
|
Title |
Method |
Mobile Phase |
Stationary Phase |
Wavelength (nm) |
|
Determination of Itopride Hydrochloride in capsule formulation[7] |
HPLC |
Methanol-Water-Triehanolamine-Glacial ascetic acid (with the proportion of 40:60:0.5:0.3,v/v/v/v) |
C18 column (4.6mm×250mm) |
258 |
|
Optimized method for the determination of itopride in human plasma[8] |
HPLC with flourometric detection |
Acetonitrile-Triethylamine-dihydrogen potassium phosphate (14.5:0.5:85,v/v/v) |
octadecylsilica column (55 mm × 4 mm, 3 μm particles), |
250/342 |
|
Chromatographic determination of Itopride Hydrochloride in the presence of its degradation products[9] |
HPLC |
Methanol-Water(70:30,v/v) |
Kromasil column [C18 (5-μm, 25 cm×4.6 mm, ID)] |
258 |
|
Simultaneous determination of Rabeprazole sodium and Itopride Hydrochloride in solid dosage form[10] |
RP-HPLC |
Acetonitrile: buffer (35:65 v/v) |
Luna C18 (5µ M, 25 cm×4.6 mm i.d) phenomenex |
266 |
|
Simultaneous determination of Esemoprazole and itopride in capsule[11] |
RP-HPLC |
Buffer(Ammonium Acetate,pH-5.5):Water:Methanol (25:15:60,v/v/v) |
Phenomenex C18 |
275 |
|
Determination of Itopride Hydrochloride in its pharmaceutical preparation and in bulk drug[12] |
HPTLC |
Methanol-Ethyl acetate-Toluene-Triethylamine (1.0:2.5:6.0:0.5,v/v/v/v ) |
Silica gel 60F 254 TLC plates |
230 |
|
Simultaneous determination of Rabeprazole sodium and Itopride Hydrochloride in solid dosage form[13] |
HPTLC |
n-butanol:toluene:ammonia (8.5:0.5:1 v/v/v) |
precoated silica gel G60F254 plate (10×10 cm) |
288 |
|
Stability indicating high performance thin-layer chromatographic method for simultaneous estimation of pantoprazole sodium and itopride hydrochloride in combined dosage form[14]
|
HPTLC |
Methanol: Water: Ammonium acetate; 4.0:1.0:0.5 (v/v/v) |
Aluminium plates precoated with silica gel 60F254 |
289 |
II.UV SPECTROSCOPIC METHOD: [15-19]
A simple, precise and economical spectrophotometric method for the estimation of Itopride Hydrochloride in pharmaceutical bulk and tablet dosage form was developed and validated. Identification was carried out using a UV- visible double beam spectrophotometer detector with working wavelength at 258nm in water and methanol medium. The method was validated with respect to its specificity, linearity range, accuracy and precision in analytical media. Itopride Hydrochloride show the maximum absorbance (λmax) at 258 nm. Simple UV spectroscopy, first derivative spectroscopy, AUC method and absorption ratio methods are reported for determination of the Itopride Hydrochloride in marketed formulation. Below in table describes the various chromatographic methods with the method description and condition which are reported on review literature.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table No.2: Summary of UV spectroscopic methods of Itopride Hydrochloride
|
Title |
Method |
Medium |
λmax (nm) |
R2 |
|
Spectrophotometric method development and validation of Itopride Hydrochloride in bulk and dosage form[15] |
Simple uv spectroscopy method |
0.1N HCL |
258 |
0.999 |
|
Estimation of Itopride Hydrochloride in Pharmaceutical Formulation[16] |
Simple uv spectroscopy method |
Distilled water |
258 |
0.9999 |
|
First derivative method |
Distilled water |
247 |
0.9998 |
|
|
AUC method |
Distilled water |
262-254 |
0.9999 |
|
|
Estimation of Itopride Hydrochloride from tablets formulations using Methyl orange reagent[17] |
New Visible Spectrophotometric method |
Distilled water
|
418.5 |
0.9991
|
|
Simultaneous estimation of Rabeprazole sodium and Itopride Hydrochloride[18] |
Q-value method |
Methanol |
284&266.4 |
0.9991& 0.9999 |
|
Simultaneous equation method |
284&258 |
0.9992& 0.9991 |
||
|
Simultaneous estimation of Pantoprazole sodium and Itopride Hydrochloride[19] |
First order derivative UV spectrophotometry |
Distilled water |
238.5& 288 |
0.9991& 0.9992 |
III.MISCELLANOUS METHODS: [18-21]
Most widely used methods are mainly HPLC, UV and HPTLC for determination of Itopride Hydrochloride in various formulation or in biological fluids but along with that other methods are also used which are seen during the literature survey. The summary of that methods are described below in table.
Table No.3: Summary of Miscellaneous methods of Itopride Hydrochloride
|
Sr.No. |
Title |
Method |
|
1 |
Stability-Indicating Spectrofluorimetric Method for Determination of Itopride Hydrochloride in Raw Material and Pharmaceutical Formulations[20] |
Spectrofluorimetric method |
|
2 |
Identification of Forced Degradation Products of Itopride by LC-PDA and LC-MS[21] |
LC-PDA & LC-MS |
|
3 |
Determination of itopride in human plasma by liquid chromatography coupled to tandem mass spectrometric detection: Application to a bioequivalence study[22] |
LC-MS/MS |
|
4 |
Simultaneous estimation of Itopride Hydrochloride and Domperidone in human plasma[23] |
LC-MS |
CONCLUSION:
The presented review highlights on various analytical methods reported on Itopride Hydrochloride and in combination with other drug. HPLC-HPTLC-UV methods were found to be most widely used. Various chromatographic conditions are presented in under Table. The faster time, high sensitivity; specificity and better separation efficiency enable HPLC to be used frequently for the determination of Itopride Hydrochloride in the comparison with the other methods. There is no doubt on the fact that these chromatographic methods are rapid and far more economical. Other methods are also useful. In this way various analytical methods for the estimation of Itopride Hydrochloride in bulk or in various matrixes like plasma, alone or in combination with other drugs is discussed. The presented information is useful for the researchers especially those involved in the formulation development and quality control of Itopride Hydrochloride in combination with other drug.
REFERENCES:
1. Ambrish T.*, Rahul M. Itopride: An updated review of its pharmacological properties and use as a prokinetic. International Journal of Institutional Pharmacy and Life Sciences, 2013; 3(2): 13-19
2. abcam.com/itopride-hydrochloride-HSR-803-ab143652.html
3. Shankar S.¹, Deepak S.¹, Sunil O.², Puspendra SN.¹*. Formulation and evaluation of Itopride Hydrochloride sustained release pellets. Innovare journal of life science,2013; 1(2): 11-16
4. Seema G. Itopride: A Novel Prokinetic Agent. JK Science, 2004; 6(2): 106-108
5. en.wikipedia.org/wiki/Itopride
6. drugs.com/cons/Itopride.html
7. Zhao Y. Hubei Provincial Institute for Drug Control, Wuhan 430064. Determination of Itopride Hydrochloride in capsule formulation by HPLC. Tianjin pharmacy, 2000-2002
8. Pavel P., Josef K., Jan M. Optimized method for the determination of itopride in human plasma by high-performance liquid chromatography with flourometric detection. Journal of Chromatography B, 2009; 877(8-9): 842-846
9. Neeraj K., Himani A., Pravin M., Janhavi RR., Kakasaheb RM and Shivajirao SK. Chromatographic determination of Itopride Hydrochloride in the presence of its degradation products. Journal of separation science, 2005; 28(13): 1566-1576
10. Rajesh S.*, Ganesh PM and Subhash CC. Development and Validation of RP-HPLC Method for the Simultaneous determination of Rabeprazole Sodium and Itopride Hydrochloride in Solid Dosage Form. E-Journal of chemistry, 2010; 7(3): 947-952
11. Rajesh KP.*, Bhuvan PR., Bhavesh HP., Laxman JP. Reverse Phase High Performance Liquid Chromatographic method for the simultaneous estimation of Esomeprazole and Itopride in Capsule. Scholar research Library, 2010; 2(1): 251-260
12. Vidya VD.¹, Ramesh TS.¹, Shashikumar NM.¹, Harsha NT.¹, Sreedevi P.¹. Determination of Itopride Hydrochloride in its pharmaceutical preparation and in bulk drug. JPC-Journal of planner chromatography- Modern TLC, 2006; 19(110): 319-323
13. Suganthi A.*, Sofiya J., and Ravi TK. Simultaneous HPTLC determination of Rabeprazole sodium and Itopride Hydrochloride in solid dosage form. Indian Journal of Pharmaceutical Science, 2008; 70(3): 366–368
14. Vineeta K.*, Deepak B., Vilasrao K. Stability indicating high performance thin-layer chromatographic method for simultaneous estimation of pantoprazole sodium and Itopride Hydrochloride in combined dosage form. Journal of Pharmaceutical Analysis, 2011; 1(4): 275–283
15. Santosh UZ.¹*, Paresh IK.¹, Jitendra WG.¹, Anantwar SP.¹, Sahebrao SB¹. Spectrophotometric method development and validation of Itopride Hydrochloride in bulk and dosage form. International Journal of Drug Delivery, 2010; 340-343
16. Gupta KR.*, R Joshi RR., Chawla RB and Wadodkar SG. UV Spectrophotometric Method for the Estimation of Itopride Hydrochloride in Pharmaceutical Formulation. E-Journal of Chemistry, 2010; 7(S1): S49-S54
17. Balram C.*¹, Anju G.¹, Sukhbir LK². Spectrophotometric method for estimation of Itopride Hydrochloride from tablets formulations using Methyl orange reagent. International Journal of Pharmacy and Pharmaceutical Sciences, 2009; 1(1): 159-162
18. Pattanayaka, Sharma Ra* & Chaturvedi SCa. Simultaneous estimation of Rabeprazole sodium and Itopride Hydrochloride. Analytical Letters, 2007; 40(12): 2288-2294
19. Deepak B., Vinash P., Vineeta K.*, Vilasrao K. Simultaneous estimation of Pantoprazole sodium and Itopride Hydrochloride in pharmaceutical dosage form by first order derivative UV spectrophotometry. Asian Journal of Pharmaceutical and Clinical Research, 2010; 3(3): 221-224
20. Mohamed I.W., Fawzia I., Manal IE., Samah AE. Stability-Indicating Spectrofluorimetric Method for Determination of Itopride Hydrochloride in Raw Material and Pharmaceutical Formulations. Journal of Fluorescence, 2013; 23(6): 1293-1300
21. Payal J.*, Suvarna B., Bhagwat AM., Vishwanath K.¹ and Jadhav RK². Identification of Forced Degradation Products of Itopride by LC-PDA and LC-MS. Indian Journal of Pharmaceutical Science, 2011; 73(3): 287–291
22. Heon WL. Ji-Hyung S., Seung-Ki C., Kyung-Tae L. Determination of itopride in human plasma by liquid chromatography coupled to tandem mass spectrometric detection: Application to a bioequivalence study. Analytica Chimica Acta, 2007; 583(1): 118–123
23. Anirbandeep B., Uttam B., Animesh G., Bappaditya C., Amlon KS., Tapan KP. LC-MS Simultaneous estimation of Itopride Hydrochloride and Domperidone in human plasma. Chromatographia, 2009; 69: 1233-1241
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









