 About Authors:
About Authors:
Vijaya Kumar.Voleti*1, Vaishnavi.V2, V.Gunasekharan1, M.S.Riyazullah1, K.Vivekanandan1
1Department of pharmaceutics, Rao’s College of Pharmacy, Nellore, Andhra Pradesh, India,
2Department of pharmaceutics, Sankar Reddy Institute of Pharmaceutical Sciences, Andhra Pradesh, India
*vijay66vvk@gmail.com
ABSTRACT:
Colon specific drug delivery has gained a more importance for the delivery at colonic region by use of various drugs to treat the both local and systemic diseases. Local diseases include Chron’s disease, ulcerative colitis, and colorectal cancer. Other serious disorders like nocturnal asthma, Hyper tension , arthritis and angina can also be cured by these techniques. Colonic delivery is a good candidature for delivery of proteins peptides and vaccines where the enzymatic degradation and the hydrolysis of proteins can be minimized and increases the systemic bioavailability. A drug should be protected from the absorption and the upper GI environment to achieve the successful colonic drug delivery. The colon specific delivery of drugs to the target receptor sites has the advantage to reduce the side effects and improves the therapeutic response. Colon specific drug delivery are being developed by taking advantage of the luminal PH conditions and the presence of microbial enzymes such as azoreductase, pectinase, dextranase…etc. This review mainly reveals on the various concepts and approaches include Prodrug, PH and time dependent systems and microbially triggered systems used in the development of colon specific drug delivery. This also focuses on the novel approaches namely Pressure controlled colonic delivery, osmotic controlled drug delivery and CODESTM. Invitro and in vivo evaluation parameters has been discussed here.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1544
INTRODUTION:
Colon is being extensively investigated as a drug delivery site. Oral Colon specific drug delivery system has been developed by means of one or more controlled released mechanisms, hardly releases drug in the upper part of GI tract, but rapidly releases drug in the colon following oral administration. CDDS is convenient for treating localized colonic diseases, i.e. ulcerative colitis, Crohn’s diseases and constipation etc., CDDS, also selectively delivers drug to the colon, but not to the upper GI tract1-3. Other potential applications include Chronotherapy, Prophylaxis of colon cancer and treatment of nicotine addiction. The treatment of IBD with anti inflammatory drugs is particularly improved by local delivery to bowel, by this technique the systemic absorption of drugs can be minimized in stomach and small intestine. The site specificity of the drugs to the target receptor sites has the potential to reduce the side effects and improve the pharmacological response. However, for successful colon specific drug delivery, many physiological barriers must be overcome; the major one is being absorption or degradation of the active drug in the upper part of the GIT. The disease state also can also potentially alter the delivery and absorption characteristics of drugs from the colon. The colon drug delivery system should protect the drug from the absorption and degradation in the stomach and small intestine, the drug should be absorbed only at the colonic site. The colon is rich in lymphoid tissue, uptake of antigens into the mast cells of colonic mucosa produces rapid local production of antibodies and this helps in efficient vaccine delivery4.
The colon is believed to be a suitable absorption site for peptides and protein drugs and is considered as an advantageous site where the bioavailability of poorly soluble drugs can be enhanced this is due to the following reasons5;
* This region is recognized as having a somewhat less hostile environment with less diversity and intensity of activity than the stomach and small intestine.
* Comparative proteolytic activity of colon mucosa is much less than that observed in the small intestine, thus CDDS protects the peptide drugs from hydrolysis, and enzyme degradation in duodenum and jejunum, and eventually releases the drug into ileum or colon which leads to greater bioavailability.
And finally, because the colon has a long residence time which is up to 5 days and is highly responsive to absorption enhancers.
Oral route is the most convenient route of administration for CDDS, other routes are also useful. Rectal administration offers the shortest route for targeting drugs to the colon. However, reaching the proximal part of colon via rectal administration is difficult. Rectal administration is uncomfortable to patients and compliance may be less than optimal.
[adsense:468x15:2204050025]
1.1.Need of colon drug delivery system6:
* Targeted drug delivery to the colon would ensure direct treatment at the disease site, lower dosing and fewer systemic side effects.
* Site-specific or targeted drug delivery system would allow oral administration of peptide and protein drugs, colon-specific formulation could also be used to prolong the drug delivery.
* Colon-specific drug delivery system is considered to be beneficial in the treatment of colon diseases.
* The colon is a site where both local or systemic drug delivery could be achieved, topical treatment of inflammatory bowel disease, e.g. ulcerative colitis or Crohn’s disease. Such inflammatory conditions are usually treated with glucocorticoid and sulphasalazine (targeted).
* A number of others serious diseases of the colon, e.g. colorectal cancer, might also be capable of being treated more effectively if drugs were targeted to the colon.
* Formulations for colonic delivery are also suitable for delivery of drugs which are polar and/or susceptible to chemical and enzymatic degradation in the upper GI tract, highly affected by hepatic metabolism, in particular, therapeutic proteins and peptides.
1.2.Advantages7:
Colon-specific drug delivery system offers the following therapeutic advantages:
· The colon is a site where both local or systemic drug delivery could be achieved, topical treatment of inflammatory bowel disease, e.g. ulcerative colitis or Crohn’s disease, Intestinal bowel syndrome (IBD).
· Minimizing extensive first pass metabolism of steroids.
· Preventing the gastric irritation produced by oral administration of NSAIDS.
· Improved therapy of diseases susceptible to diurnal rhythm.
· Delayed release of drugs to treat angina, asthma and rheumatoid arthritis.
· Potential for oral delivery of proteins, peptides and other GI liable drugs.
· By producing the ‘friendlier’ for peptides and proteins when compared to upper GI tract.
· The therapy of various disease conditions can be improved by colon specific drug delivery systems employing various mechanisms of release, which is shown in following table.
Table 1: Colon targeting diseases, Drugs and sites8:
|
Target Sites |
Disease conditions |
Drugs and active agents |
|
TOPICAL ACTION |
Inflammatory Bowel disease, Irritable bowel Syndrome, Chronic Pancreatitis, and Crohn’s disease. |
Hydrocortisone, Budenoside, Prednisolone, Mesalamine and Balsalazide. |
|
LOCAL ACTION |
Pancreoctomy and Crystal fibrosis, Colorectal cancer |
Digestive enzymes supplements, 5-Flourouracil. |
|
SYSTEMIC ACTION |
To prevent gastric irritation To prevent first pass metabolism for orally ingested drugs Orally delivered peptides |
NSAIDS Steroids Insulin Typhoid |
Delayed systemic absorption of drugs via colonic delivery is advisable for Chronotherapy of diseases such as asthma, hypertension, cardiac arrhythmias, rheumatoid arthritis or inflammation, which are affected by circadian rhythms. These diseases are characterized by night time or early morning symptoms. These types of approaches are beneficial for nocturnal release of drug, which in turn may provide considerable relief to the patients while they are resting.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
The mode of drug release from colon-targeted biopolymer systems can include one or more of the following mechanisms9:
1. Diffusion
2. Polymer erosion
3. Microbial degradation
4. Enzymatic degradation (mammalian and/or bacterial)
In addition, drug solubility and formulation of polymer mixes play important roles in determining the extent of drug delivery and release in the colon.
Two broad categories of biopolymers have been employed for formulating colonic systems:
(1) Biodegradable and
(2) Non biodegradable polymers.
1.3.Limitations10:
To achieve successful colon targeting it should overcome the following limitations (Jack et al., 2006).
· The location at the distal portion of the alimentary canal, the colon is difficult to access.
· Successful delivery requires the drug to be in solution before it arrives in the colon, but the fluid content in the colon is lower and more viscous than in upper GIT, which is the limiting factor for poorly soluble drugs.
· Lower surface area and relative tightness of the tight junctions in the colon can restrict drug transport across the mucosa in to the systemic circulation.
1.4.General considerations for designing of colon specific formulations11:
To achieve a desired therapeutic action of dosage form, it is necessary to design a suitable formulation with suitable qualities. In general, delayed release dosage forms are designed to provide a burst release or a sustained/ prolonged release once they reach colon.
Various factors includes are
· Pathology and pattern of diseases, especially the affected parts of lower GIT or, Physiology and physiological composition of the healthy colon if the formulation is not intended for localized treatment.
· Physicochemical properties and biopharmaceutical properties of the drug such as solubility, stability and permeability at the intended site of delivery, and the desired release profile of the active ingredient.
Formulation of drugs for colon specific delivery requires careful consideration of dissolution of and / or release rate in the colon fluids. Due to the presence of less fluid content in large intestine than in small intestine the dissolution and release rate from the formulations decreases. The poor dissolution and release rate may in turn lead to lower systemic availability of drugs. These issues could be more problematic when the drug candidate is poorly water soluble and / or require higher doses for therapy. Consequently, such drugs need to be delivered in pre solubilized form, or formulation should be targeted for proximal colon, which has more fluid than in the distal colon. Aside from drug solubility, the stability of the drug in the colonic environment is a further factor that warrants attention. The drug could bind in a nonspecific manner to dietary residues, intestinal secretions, mucus or general fecal matter, thereby reducing the concentration of free drug. Moreover, the resident micro-flora could also affect colonic performance via degradation of the drug.
1.5.Anatomy and physiological considerations12:
The GI tract is divided into stomach, small intestine and large intestine. The large intestine extending from the ileocecal junction to the anus is divided in to three main parts. These are the colon, the rectum and anal canal.
The entire colon is about 5 feet (150 cm) long, and is divided in to five major segments. Peritoneal folds called as mesentery which is supported by ascending and descending colon. The right colon consists of the caecum, ascending colon, hepatic flexure and the right half of the transverse colon and the values were shown in Table 2. The left colon contain the left half of the transverse colon, descending colon, splenic flexure and sigmoid. The rectum is the last anatomic segment before the anus. The human intestine and colon were shown in Figure1 and Figure 2 respectively.
The major function of the colon is the creation of suitable environment for the growth of colonic microorganisms, storage reservoir of fecal contents, expulsion of the contents of the colon at an appropriate time and absorption of potassium and water from the lumen. The absorptive capacity is very high, each about 2000ml of fluid enters the colon through the ileocecal valve from which more than 90% of the fluid is absorbed. On average, it has been estimated that colon contains only about 220 gm of wet material equivalent to just 35 gm of dry matter. The majority of this dry matter is bacteria. Colon targeted drug delivery would ensures direct treatment at the disease site, lower dosing and less systemic side effects. In addition to restricted therapy, the colon can also be utilized as a portal for the entry of drugs into the systemic circulation. For example, molecules that are degraded/poorly absorbed in the upper gut, such as peptides and proteins, may be better absorbed from the more benign environment of the colon. . Overall, there is less free fluid in the colon than in the small intestine and hence, dissolution could be problematic for poorly water-soluble drugs. In such instances, the drug may need to be delivered in a pre-solubilized form, or delivery should be directed to the proximal colon, as a fluid gradient exists in the colon with more free water present in the proximal colon than in the distal colon. Aside from drug solubility, the stability of the drug in the colonic environment is a further factor that warrants attention. The drug could bind in a nonspecific manner to dietary residues, intestinal secretions, mucus or general faecal matter, thereby reducing the concentration of free drug. Moreover, the resident micro-flora could also affect colonic performance via degradation of the drug.
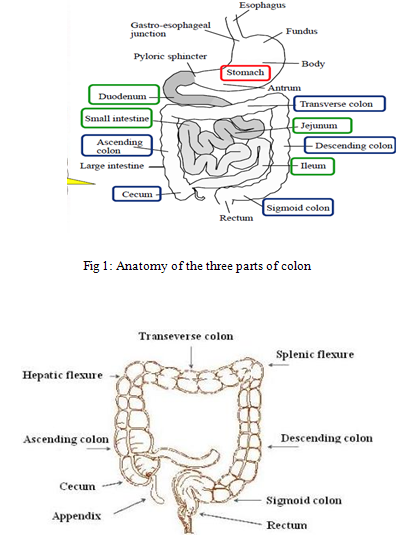
Fig 2. Main features of the colon
Different properties of GIT were given in (Table 2) and different enzymes present in colon, which are responsible for microbial degradation, were reported by Vincent et al (2002).
Table2. Properties of Gastro Intestinal Tract13:
|
Region of GIT |
Property |
Measured value |
|
Total GIT |
Surface area |
2-106 cm2 |
|
Small intestine -Duodenum -Jejunum -Ileum |
Length |
20-30 cm 150-250 cm 200-350 cm |
|
Large intestine Cecum -Ascending colon -Descending colon -Transverse colon -Sigmoid colon -Rectum -Anal canal |
Length |
6-7 cm 20 cm 45 cm 30 cm 40 cm 12 cm 3 cm |
|
Small intestine Large intestine |
Internal diameter |
3-4 cm 6 cm |
|
Stomach Duodenum Jejunum Ileum Colon Rectum |
PH |
1 3.5 5-7 6-7 7 5.5-7 7 |
|
Colon -Right -Mid -Left |
Redox potential |
- 415 - 400 - 380 |
1.6.Gastrointestinal transit14
Gastric emptying of dosage form is highly variable and depends primarily on whether the subject is fed or fasted and on the properties of the dosage form such as size and density. The transit times of dosage forms in tract are shown in Table 3.
Table 3: Gastrointestinal Transit time of contents
|
Organ |
Transit Time (hr) |
|
Stomach Small intestine Large intestine |
<1(fasting) >3(fed) 3-4 20-30 |
1.7. PHARMACEUTICAL APPROACHES FOR CDDS15
Various pharmaceutical approaches that can be exploited for the development of colon targeted drug delivery systems are given below:
Approaches used for site specific drug delivery are –
Primary approaches for CDDS
- PH sensitive polymer coating drug delivery to colon.
- Delayed (time controlled release system) release drug delivery to colon.
- Microbially triggered drug delivery to colon.
i) Prodrug approach for drug delivery to colon.
ii) Azo – polymeric approach for drug delivery to colon.
iii) Polysaccharide based approach for drug delivery to colon.
Newly developed approaches for CDDS
- Pressure controlled drug delivery system (PCDCS)
- CODESTM (a novel colon targeted delivery system)
- Osmotic controlled drug delivery to colon (OROS – CT)
- Pulsincap system
- Port system
- Time clock system
- Chronotropic system
- Colal – Pred system
- Target technology
- Ticking capsule
- Enterion capsule technology
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
1.7.1. PRIMARY APPROACHES
1.7.1.1. PH Dependentsystems:
The pH-dependent systems exploit the generally accepted view that pH of the human GIT increases progressively from the stomach (pH 1-2 which increase to 4 during digestion), small intestine (pH 6 - 7) at the site of digestion and it increases to 7-8 in the distal ileum. The gamma scintigraphy technique becomes most popular technique to investigate the gastrointestinal performance of pharmaceutical formulations. The pH sensitive polymers (given in Table 4) which will produce delayed release and also give protection from gastric fluids16.
Table 4: List of Enteric Polymers used in the development of Modified-Release Formulations for Colonic drug Delivery systems
|
Enteric Polymers |
Optimum pH for dissolution |
|
Polyvinyl acetate phthalate (PVAP) |
5.0 |
|
Cellulose acetate tri melitate (CAT) |
5.5 |
|
Hydroxypropyl methylcellulose phthalate (HPMCP) |
> 5.5 |
|
Hydroxy propyl methylcellulose acetate succinate (HPMCAS) |
> 6.0 |
|
Methacrylie acid copolymer, Type C (Eudragit L100-55) |
> 6.0 |
|
Methacrylic acid copolymer dispersion (Eudragit L30D-55) |
> 5 |
|
Methacrylic acid copolymer, Tyep A |
> 6.0 |
|
(Eudragit®L-100 and Eudragist L12,5) |
_ |
|
Cellulose acetate phthalate (CAP) (Aquateric) |
6.0 |
|
Methacrylic acid copolymer, Type B |
> 7.0 |
|
(Eudragist S-100 and Eudragit S12,5) |
_ |
|
Eudragit FS30D |
> 7.0 |
|
Shellac (MarCoat 125 &125N) |
7.0 |
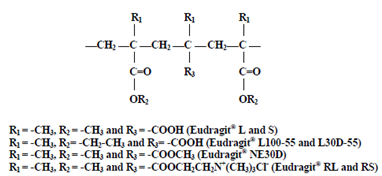
Fig 3: Chemical structure of Eudragit
The selected polymers to colon targeting should be able to withstand the pH of the stomach and small intestine. Methacrylic acid esters most commonly used polymers for colon targeting because they are soluble at above pH 6. The ideal polymer should be able to withstand the lower pH of the stomach and of the proximal part of the small intestine but able to disintegrate at neutral or shortly alkaline pH of the terminal ileum and preferably at ileocecal junction. Eudragit-L and Eudragit S are widely used in the colon targeting because Eudragit L is soluble at pH 6 or above and Eudragit S is soluble at pH 7 or above and the combination of these polymers give the desirable release rates. The polymers of this class are insoluble at low PH levels but become increasingly soluble as PH rises. The polymers of this class protect the formulation from the acidic PH and proximal small intestine, it may start to dissolve in the lower small intestine, and the site specificity of formulations can be poor. PH-sensitive hydro gels were prepared for colonic delivery of therapeutic peptides, proteins. New pH-sensitive glycopolymers were developed by free radical polymerization of Methacrylic acid and 6-hexandiol diacrylate and 6- hexandiol propoxylate diacrylate. Colon targeted drug delivery systems based on methacrylic resins has described for insulin, prednisolone, quinolones, salsalazine, cyclosporine, beclomethasone dipropionate and naproxen, Khan et al. prepared lactose-based placebo tablets and coated using various combinations of two methacrylic acid polymers, Eudragit L100-55 and Eudragit 100 by spraying from aqueous systems. The same coating formulations are then applied on tablets and evaluated for in vitro dissolution rates under various conditions.
1.7.1.2. Time-dependent systems:
Time dependent systems are very promising type of drug release systems. The dosage forms also applicable to colon targeting dosage forms by prolonging the lag time of about 5 to 6 hours. However the disadvantages of this system are17:
1. Gastric emptying time varies markedly between subjects or in a manner dependent on type and amount of food intake.
2. Gastrointestinal movement, especially peristalsis or contraction in the stomach would result in change in gastrointestinal transit of the drug.
3. Accelerated transit through different regions of the colon has been observed in patients with the IBD, the carcinoid syndrome and diarrhea, and the ulcerative colitis.
Therefore, time dependent systems are not ideal to deliver drugs to the colon specifically for the treatment of colon related diseases.Colon targeting could be achieved by incorporating a lag time into formulation equivalent to the mouth to colon transit time. The basic principle involved in the system is the release of drug from dosage form should be after a predetermined lag time to deliver the drug at the right site of action at right time and in the right amount. Enteric coated time-release press coated (ETP) tablets, are composed of three components, a drug containing core tablet (rapid release function), the press coated swellable hydrophobic polymer layer (Hydroxy Propyl cellulose layer (HPC), time release function) and an enteric coating layer (acid resistance function).The tablet does not release the drug in the stomach due to the acid resistance of the outer enteric coating layer. After gastric emptying, the enteric coating layer rapidly dissolves and the intestinal fluid begins to slowly erode the press coated polymer (HPC) layer. When the erosion front reaches the core tablet, rapid drug release occurs since the erosion process takes a long time as there is no drug release period (lag phase) after gastric emptying. The duration of lag phase is controlled either by the weight or composition of the polymer (HPC) layer.
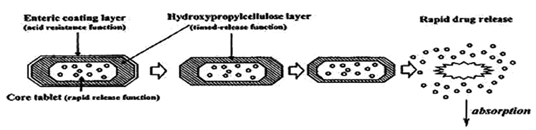
Fig 4:Design of enteric coated timed-release press coated tablet (ETP Tablet)
A nominal lag time of five hours is usually considered sufficient to achieve colon targeting. In this method the solid dosage form coated with different sets of polymers (listed in Table 6) and the thickness of the outer layer determines the time required disperse in aqueous environment Hydroxy Propyl Methyl Cellulose (HPMC) compression coated tablets of 5-fluorouracil were studied for colon drug delivery that based on time-dependent approach. In this, the core tablet was prepared by wet granulation method and then coated with 50% of HPMC/lactose coat powder by compression-coating method. Drug release characteristics were evaluated in distilled water by using a Chinese pharmacopoeia rotatable basket method.
1.7.1.2.1. Pulsatile system
In recent years considerable attention has been focused on the development of pulsatile drug delivery system. Delivery system with pulsatile release pattern has gained most popular form of controlled drug delivery system because conventional systems with a continuous release are not ideal. Oral controlled drug delivery systems are generally used due to convenient dosage form and it also releases drug in constant or variable rates. In these system drug release generally occurs within therapeutic window for prolong period of time. Hence these systems show sustained release of drug from dosage form. Pulsatile release systems are formulated to undergo a lag-time of predetermined span of time of no release, followed by a rapid and complete release of loaded drugs18. The approach is based on the principle of delaying the time of drug release until the system transits from mouth to colon. A lag-time of 5 hours is usually considered sufficient since small intestine transit is about 3-4 hours, which is relatively constant and hardly affected by the nature of formulation administered.
Advantages of Pulsatile Drug Delivery System
- Extended daytime or night time activity
- Reduced side effects
- Reduced dosage frequency
- Reduction in dose size
- Improved patient compliance
- Lower daily cost to patient due to fewer dosage units are required by the patient in therapy.
- Drug adapts to suit circadian rhythms of body functions or diseases.
- Drug targeting to specific site like colon.
- Protection of mucosa from irritating drugs.
- Drug loss is prevented by extensive first pass metabolism.
The oral controlled drug delivery system with continuous release does not show suitability in various conditions of the body which require pulsatile release of drug defined as “a pulsatile release profile” and is characterized by a time period of no release (lag time) followed by a rapid and complete drug release of drug from dosage form. Conditions requiring pulsatile release include a number of hormones like rennin, aldosterone and cartisole which shows daily fluctuation in their blood levels. These changes are generally known as circadian rhythm which is responsible for changes in many functions of the body like activity of liver enzyme, blood pressure, and intraocular pressure etc PH, gastric acid secretion in stomach, gastric emptying and gastric intestinal blood transfusion. Various diseases are also dependent on the circadian rhythm for example acute myocardial insufficiency occurs most commonly around 4.00 P.M. and Epileptic seizures have the highest incidence in the morning, such conditions demands consideration of diurnal progress of disease rather than maintaining constant plasma drug level. In these conditions delivery system should be administered at night but it should release drug at early morning time. Some other diseases are bronchial asthma, angina pectoris, rheumatic disease, ulcer and hypertension also required time dependent delivery. Drugs responsible for producing biological tolerance also require pulsatile release. These systems prevent their continuous presence at the bio phase. It releases drug after lag time (time at which drug is required by the body). For drugs required to be targeted in colonic region (distal organ) the delivery system should prevent release of drug in the upper two third portions in gut. Drug with idiosyncratic pharmacokinetics or pharmacodynamics or drugs with extensive first pass metabolism or which show potential food interaction require pulsatile release of the drug19. Some drugs induce nausea or vomiting or some cause gastric irritation or some undergo degradation in gastric acid medium, all such drug requires drug release after lag time. Pulsed fashion can be achieved by the enteric coating of delivery system.
All above conditions are required for chronotherapeutic (i.e. precisely time therapy). To accomplish the objectives and advantages of chronotherapeutic, time controlled pulsatile drug delivery devices are required they show releasing the right amount at the right time.
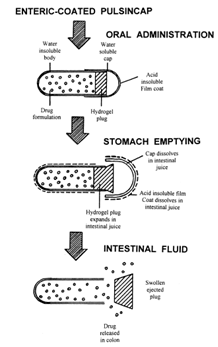
Fig 5: Enteric coated pulsing cap
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
1.7.1.3. Micro flora activated system
A large number of anaerobic and aerobic bacteria are present the entire length of the human GI tract. Over 400 distinct bacterial species have been found, 20- 30% of which are of the genus bacteroids7. The upper region of the GIT has a very small number of bacteria and predominantly consists of gram positive facultative bacteria. The rate of microbial growth is greatest in the proximal areas because of high concentration of energy source20.
Table 5: The human gastro-intestinal flora:
|
|
Stomach |
Jejunum |
Ileum |
Feces |
|
Total bacterial count |
0-103 |
0-105 |
103-107 |
1012-1012 |
|
Anaerobic bacteria |
||||
|
E.Coli |
0-102 |
0-103 |
102-106 |
104-1010 |
|
Strepto cocci |
0-103 |
0-104 |
102-106 |
105-1010 |
|
Staphylo cocci |
0-102 |
0-103 |
102-105 |
104-107 |
|
Lactobacilli |
0-103 |
0-104 |
102-105 |
106-1010 |
|
Fungi |
0-102 |
0-102 |
102-103 |
102-106 |
|
Anaerobic bacteria |
||||
|
Bacteroids |
Rare |
0-102 |
103-105 |
1010-1012 |
|
Bifid bacteria |
Rare |
0-103 |
103-107 |
108-1012 |
The metabolic activity of micro flora can be modified by various factors such as age, GI disease, and intake of drug and fermentation of dietary residues
1.7.1.3.1. Prodrug approach:
A Prodrug is a pharmacologically inactive derivative of a parent compound that requires enzymatic transformations in order to release the drug and that has improved release properties over the parent compound. Formulation of Prodrug has improved delivery properties over the parent compound. The choice of carrier is largely determined by the functional group available on the drugs. There are at least three factors should be optimized for the site specific delivery of drugs by using the Prodrug approach21.
1. The Prodrug must to reach the target for the site of action as early as possible, and uptake from the site must be fast and essentially perfusion rate limited.
2. Once the drug reached to the site, Prodrug must be selectively liberated to the active drug relative to its conversion at other sites.
3. Once selectively liberated at the site of action, the active drug must be somewhat retained by the tissue.
The problem of stability of certain drugs from the adverse environment of the upper GIT can be eliminated by Pro drugs formation, which is converted into parent drug molecule once it reaches to colon. Enzymes produced by bacterial flora, which are responsible for the cleavage of Prodrug, are listed in Table 6
Table 6: Enzymes in Colon:
|
Reducing enzymes |
Hydrolytic enzymes |
|
Nitroreductase Azoreductases N-oxide reductase Sulphoxide reductase Hydrogenase |
Esterases Amidases Glycosidases Glucuronidase Sulfatase |









