 About Authors:
About Authors:
*S.B.Muthu Vadivel, R.Suresh Kumar, A.Tamil Selvan, R.Suthakaran
Department of Pharmaceutical Analysis and Quality Assurance
Teegala Ram Reddy College of Pharmacy
Saroor nagar, Meerpet, Hyderabad – 97.
*muthuvadivelanalyst@gmail.com
Abstract
Mass spectrometry has been applied to almost every area of research being pursued today. Studies of diverse subjects, such as cancer research, identification of drugs, forensic analysis, atmospheric end-water environmental analysis, combustion, and lasers have benefited from mass spectrometry. In some of these studies, the mass spectrometer is used both as a chemical reactor and as an analytical instrument. Because of these diverse applications, no person or group can completely review the field of mass spectroscopy. This chapter discusses instrumental designs and techniques, ionization processes, ion–molecule reactions, high-temperature systems, and sampling of reactive species. Any tandem mass spectroscopy has four basic components: (I) a system by which the sample to be studied is introduced into the instrument, (II) an ion source where ions that are characteristic of the sample are produced, (III) an analyzer region where the ion beam is sorted into its various mass-to-charge ratios, and (IV) a detector system where the separated ion beams are collected and by some method, rendered observable.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1581
INTRODUCTION
Analysis of biological sample is the measurement of drug concentration in various biological fluids. The main phases that comprise the analysis of biological sample are method development, method validation and unknown sample analysis (method application).
Bioanalytical methods is the process for quantitative determination of drugs and their metabolites in biological matrix (plasma, urine, saliva, serum etc), It plays an in numerous role to generate the pharmacokinetic data by measuring bioavailability and hence bioequivalence.1 The development of bioanalytical method is of paramount importance during the process of drug discovery and development, culminating in a marketing approval.
The reliability of bioanalytical methods is a matter of great importance in clinical and toxicological field because it is the prerequisite for correct interpretation of toxicological endings. Unreliable results might not only be contested in court, but could also lead to wrong treatment of the patient. Hence, whatever way the analysis is done it must be checked to see whether it does what it was intended to do. Each step in the method must be investigated for the extent to which environment, matrix, or procedural variables can affect on the estimation of analyte in the biological media from the time of collection up to the time of analysis 2, due to these facts importance of validation, at least of routine analytical methods, can therefore hardly be overestimated.
Moreover due to increased interdependence among countries in recent times it has become necessary for results of many methods to be accepted internationally. Consequently, to assure common level of quality, the need for and use of validated methods has increased 3. Bio-analytical method is used to estimation of drugs in biological fluids for identification, isolation and quantification of different drugs and their metabolites from biological fluids .The ultimate is of detection techniques that should be highly sensitive and specific for the quantification of drugs.Both HPLC and LC-MS/MS are used for detection. HPLC itself coupled with UV, PDA or fluorescence detector is generally used but it does not give the high sensitivity as required by some of the potent, low dose drugs.The main advantages of LCMS-MS include low detection limits up to picogram, the ability to generate structural information, the requirement of minimal sample treatment and the possibility to cover a wide range of analyte differing in their polarities.4
Liquid chromatography linked to tandem mass spectrometry has played an important role in pharmacokinetics and metabolism studies at various drug development stages hence it is introduced to the pharmaceutical industry. Newly introduced techniques such as “ultra performance liquid chromatography” with small particles (sub-2µm) and monolithic chromatography offer improvements in speed, resolution and sensitivity compared to conventional chromatographic techniques.5
[adsense:468x15:2204050025]
IONIZATION TECHNIQUES6, 7
Different types of ionization techniques ESI, APCI, APPI are most commonly used . The mass spectrometric ionization techniques of electron ionization (EI) 20 and chemical ionization (CI) 21 required the analyte molecules to be present in the gaseous form. Hence there is the need for an interface that will eliminate the solvent and generate gas phase ions. Moreover the interface is the one which connect the LC to MS. Following diagram provides an idea in selection of proper ionization source.
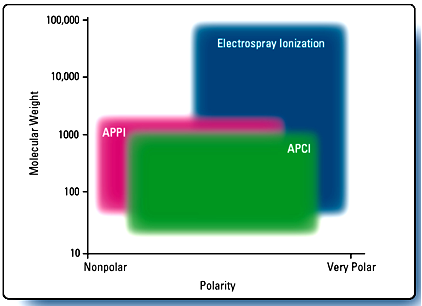
Fig- Ionization Techniques
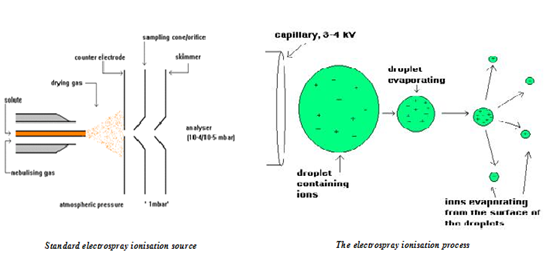
ELECTROSPRAY IONIZATION (Turbo spray) 7, 8
Electrospray generates analyte ions from solution before the analyte reaches the mass spectrometer. The LC eluent is sprayed (nebulized) into a chamber at atmospheric pressure in the presence of a strong electrostatic field and heated drying gas. The electrostatic field causes further dissociation of the analyte molecules. The heated drying gas converted the solvent in to droplets to evaporate. As the droplets shrink, the charge concentration in the droplets increased. Eventually, the similar charge ion repulsive force exceeds the cohesive forces and ions are desorbed into the gas phase. These ions are attracted to and pass through a capillary sampling orifice into the mass analyzer.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
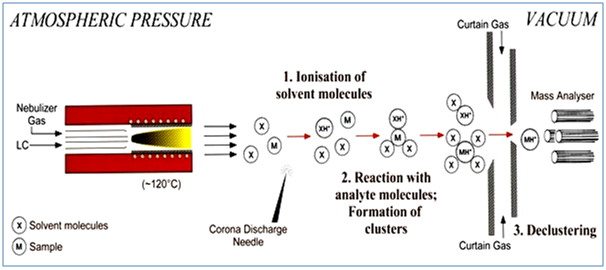
4.1.2. ATMOSPHERIC PRESSURE CHEMICAL IONIZATION8, 9
In APCI, the LC eluent is sprayed through a heated (typically 250°C – 400°C) vaporizer at atmospheric pressure. The heat vaporizes the liquid. The resulting gas-phase solvent molecules are ionized by electrons discharged from a corona needle. The solvent ions then transfer charge to the analyte molecules through chemical reactions (chemical ionization).
The Analyte ions pass through a capillary sampling orifice into the mass analyzer. APCI is applicable to a wide range of polar and nonpolar molecules. It rarely results in multiple charging so it is typically used for molecules less than 1,500µ. Due to this, and because it involves high temperatures, APCI is less well-suited than electrospray for analysis of large biomolecules that may be thermally unstable. APCI is used with normal-phase chromatography more often than electrospray is because the analytes are usually nonpolar.
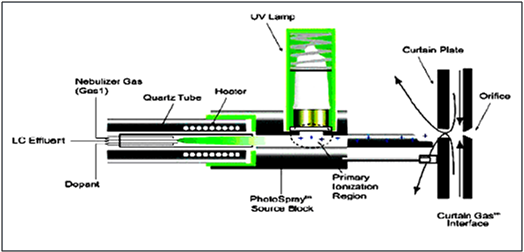
4.1.3. ATMOSPHERIC PRESSURE PHOTO IONIZATION9, 10
Atmospheric pressure photo ionization (APPI) for LC-MS/MS is a relatively new technique. As in APCI, a vaporizer converts the LC eluent to the gas phase. A discharge lamp generates photons in a narrow range of ionization energies. The range of energies is carefully chosen to ionize as many analyte molecules as possible while minimizing the ionization of solvent molecules. The resulting ions pass through a capillary sampling orifice into the mass analyzer.
APPI is applicable to many of the same compounds that are typically analyzed by APCI. It shows particular promise in two applications, highly nonpolar compounds and low flow rates (<100 µl/min), where APCI sensitivity is sometimes reduced.
In all cases, the nature of the analyte(s) and the separation conditions has a strong influence on which ionization technique: electrospray, APCI or APPI will generate the best results. The most effective technique is not always easy to predict.
MASS ANALYZER (Quadrupole) 10
A quadrupole mass analyzer consists of four parallel rods arranged in a square. The analyte ions are directed down the center of the square. Voltages applied to the rods generate electromagnetic fields. These fields determine which mass-to-charge ratio of ions can pass through the filter at a given time. Quadrupole tend to be the simplest and least expensive mass analyzers.
In LC-MS/MS triple quadrupole are used denoted as Q1, Q2 and Q3. Among this three first will selectively scan parent ion where as 2nd causes the collusion deactivation of unwanted fragment .At last Q3 determine the most intense daughter ion.
Quadrupole mass analyzers can operate in various modes:
- Scanning (scan) mode: The mass analyzer monitors a range of mass-to-charge ratios
- Selected ion monitoring (SIM) mode: SIM mode is significantly more sensitive than scan mode but provides information about fewer ions because the mass analyzer monitors only a few mass to- charge ratios
- Selected/multiple reaction monitoring, MRM Mode: This mode is applicable when there is more than one quadrupole. Both of the analyzers are static in this case as user-selected specific ions are transmitted through the first analyzer and user-selected specific fragments arising from these ions are measured by the second analyzer. The compound under scrutiny must be known and have been well-characterized previously before this type of experiment is undertaken
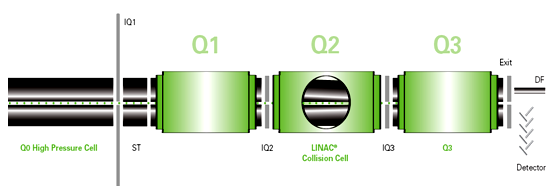
Fig- Qudrapole mass analyzer 4.3. APPLICATIONS OF LC-MS/MS
* Peptide mapping
* Selective detection of compounds in a complex mixture
* Efficient analysis of biological samples
* To identify degradation products in stability studies
* Identification of metabolites
* Quantification of compounds in biological matrix.
5.0. QUANTITATIVE ANALYSIS
Three methods are generally used for quantitative analysis. They are the external standard method, the internal standard method and the standard addition method.
5.1. EXTERNAL STANDARD METHOD
The external standard method involves the use of a single standard or up to three standard solutions. The peak area or the height of the sample and the standard used are compared directly or the slope of the calibration curve based on standards that contain known concentrations of the compounds of interest.
5.2. INTERNAL STANDARD METHOD
A widely used technique of quantitation involves the addition of an internal standard to compensate for various errors. In this approach, a known compound of a fixed concentration is added to the known amount of samples to give separate peaks in the chromatograms, to compensate for the losses of the compounds of interest during sample pre-treatment steps. Any loss of the component of interest will be accompanied by the loss of an equivalent fraction of internal standard. The accuracy of this approach obviously dependents on the structural equivalence of the compounds of interest and the internal standard.
The requirements for an internal standard must
¨ Give a completely resolved peak with no interferences
¨ Elute close to the compound of interest
¨ Behave equivalent to the compounds of interest for analysis like pretreatments, derivative formations, etc
¨ Be added at a concentration that will produce a peak area or peak height ratio of about unity with the compounds of interest
¨ Not be present in the original sample
¨ Be stable, unreactive with sample components, column packing and the mobile phase and
¨ Be commercially available in high purity
Free from Drug-drug interaction
REFERENCES
1. Bressolle F, Bromet-Petit M, and Audran M (1996) Validation of liquid chromatographic and gas chromatographic methods. Applications to pharmacokinetics. J Chromatography B 686: 3-10.
2. Evans.G, Handbook of Bioanalysis and Drug metabolism,8-69,(2004)
3. Guidance for Industry: Bioanalytical Method Validation, US Department of Health and Human Services, US Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Rockville, May 2001.
4. Pranay W, Brijesh k, Dr. Anil B. Bioanalytical Method Development –Determination of Drugs in Biological Fluids. J Pharm Sci and Technol; 2010: Vol. 2 (10):333-347
5. Raymond N, Leimin F, Matthew JR, Tawakol A. Recent advances in high-throughput quantitative bioanalysis by LC–MS/MS. J of Pharm and Biomed Anal 2007;( 44) :342–355
6. Guidance for Industry-Bioanalytical Method Validation, US Department of Health and Human services, Food and Drug Administration, Center for Drug and Evaluation and Research(CDER) and Center for Veterinary Medicine(CVM), (2001),http://fda.gov/cder/guidance/index.htm
7. Evans.G, Handbook of Bioanalysis and Drug metabolism,8-69,(2004)
8. W.M.A. Niessen, Liquid Chromatography-Mass spectrometry,2nd edition Vol-79,31-69,287-290,337-343,(1999)
9. Cole R, ESI-MS Fundamental instrumental method of chemical analysis.
10. Merrit W,Settle D Instrumental methods of Chemical analysis, 465, 502, 513,-529, 580-592,(2000)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









