About Author:
Parul Lal
Product Manager
Sapient Laboratories PVT. LTD
parullal2011@gmail.com
Abstract:
Diabetes Mellitus is a metabolic disorder which results from defects in insulin secretion, insulin action, or both, further characterized by hyperglycemia, and causes long term damage and failure of various organs. It is estimated that 366 million people had Diabetes Mellitus in 2011; by 2030 this would have risen to 552 million. Many oral hypoglycaemic agents are marketed nowadays. DPP-IV Inhibitors or Gliptin, a new oral hypoglycaemic class, they work by promoting insulin secretion by stimulating Incretins; GLP-1 (Glucagon Like Peptide-1) and GIP (Glucose -dependent insulinotropic peptide) and inhibiting DPP-IV enzyme which deactivates incretins. DPP-IV Inhibitors produce no weight gain and may have long term beneficial effects on beta-cell function and mass and so far they have fewer gastrointestinal effects. Now this drug class is available, we will discuss the disease pathology and mechanism of action of DPP-IV inhibitors, efficacy of this new class of oral hypoglycaemic agents.
Reference Id: PHARMATUTOR-ART-1964
Introduction
The prevalence of diabetes, constituted chiefly by type 2 diabetes (T2D), is a global public health threat. The prevalence among adults aged 20-70 years is expected to rise from 285 million in 2010 to 438 million by the year 2030(1). Diabetes Mellitus caused 4.6 million deaths in 2011(2). The incidence of type 2 DM varies substantially from one geographical region to the other as a result of environmental and lifestyle risk factors(3).
PATHOPHYSIOLOGY OF TYPE 2 DIABETES MELLITUS
The pathophysiology of type 2 diabetes mellitus is characterized by peripheral insulin resistance, impaired regulation of hepatic glucose production, and declining β-cell function, eventually leading to β -cell failure.
The primary events are believed to be an initial deficit in insulin secretion and, in many patients, relative insulin deficiency in association with peripheral insulin resistance(4,5).
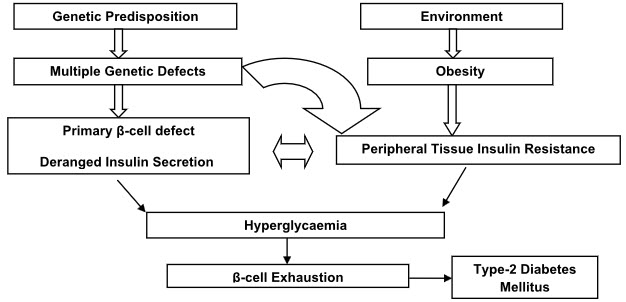
Figure 1: Pathophysiology of Type 2 Diabetes Mellitus
PHARMACOTHERAPY USED IN TYPE 2 DIABETES MELLITUS
Current therapeutic agents available for type 2 diabetes mellitus include sulfonylureas and related compounds, biguanides,thiazolidinediones, α-glucosidase inhibitors and insulin.The most common side effect of sulfonylurea is hypoglycaemia, which though usually mild to moderate, can cause fatal complication(6,7). The main adverse effects with thiazolidinediones are weight gain (dose-dependent) of 1-4kg after 6 months of treatment(8).
So as to overcome the side-effects of Oral hypoglycaemic drugs a new class of drugs called Gliptins orDipeptidyl Peptidase 4 (DPP-4) inhibitorswas introduced. These drugs work by prolonging the action of gut hormones called incretins, which boost insulin levels. The greatest advantage of the gliptins appears to be their ability to stimulate insulin production with little risk of corresponding hypoglycemia. Gliptins act by two way which are as follows:
A. INCRETINS PROMOTE INSULIN SECRETION
This is known for more than 20 years that insulin levels rise considerably higher in response to an oral glucose load than to an intravenous glucose infusion, even though the plasma glucose concentrations may be similar(9). This phenomenon involves a myriad of neural and nutritional factors, but the gut hormones glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP) appear to be key. These peptides—called incretins—have a high degree of homology, and both promote insulin secretion. However, GLP-1, produced by the L cells of the ileum and colon, inhibits glucagon secretion and slows gastric emptying, whereas GIP, secreted from the K cells of the duodenum, has no effect on glucagon and little effect on gastric emptying. Both peptides appear to promote pancreatic beta cell growth and survival, (10,11)an effect that in theory might allow us to slow the progressive loss of insulin secretory capacity in type 2 diabetes. Furthermore, the effect of GLP-1 on insulin secretion depends on the plasma glucose concentration, with a greater insulin secretory effect at higher glucose levels and minimal effect at euglycemic levels (12). This phenomenon suggests that drugs that boost GLP-1 activity should not cause the troublesome hypoglycemia typically seen in patients taking insulin, insulin secretagogues, sulfonylureas, or the meglitinides repaglinide or nateglinide.
B. INHIBITION OF DPP-4 BOOSTS INCRETIN ACTION
The challenge for creating treatments that take advantage of the beneficial effects of GLP-1 and GIP is that they have very short physiologic half-lives, i.e., less than 10 minutes. GLP-1 and GIP both have two N-terminal amino acids that are quickly cleaved by DPP- 4 (13) an enzyme present in the circulation (14)and on endothelial cells (15).
By inhibiting the cleaving action of DPP-4, the gliptins can prolong the half-life of endogenous GLP-1, increasing its physiologic effects.
Preclinical studies have shown evidence of improved beta cell growth and survival with DPP-4 inhibitor treatment, to an extent similar to that reported with thiazolidinediones, whereas sulfonylureas show no evidence either of increase in beta cells or of improved intrinsic beta cell secretory function in these models.
As a new class of oral anti-diabetic drug DPP IV inhibitors have important advantages over other currently available anti- diabetic agents. Compared with currently available insulin secretagogues, DPP IV inhibitors, by enhancing biologically active (intact) GLP-1, increase insulin secretion and suppress glucagon secretion in a glucose-dependent manner. DPP IV inhibitors, produce no weight gain and may have long-term beneficial effects on beta-cell function and mass although, this is only demonstrated so far in animal models. Compared to the GLP-1 analogues (incretin mimetics), DPP IV inhibitors have the advantage of being administered orally and so far with fewer gastrointestinal side effects (nausea and vomiting). In addition to elevation of active GLP-1, DPP IV inhibition does elevate the concentrations of biologically active GIP, in spite of an impaired effect of GIP in patients with type 2 diabetes.(16, 17)There is a limit to the levels of intact GLP-1 that can be obtained with the DPP IV inhibitors: 1) the secretion of GLP-1 is impaired in patients with type 2 diabetes, limiting the levels of intact GLP-1 which can be obtained with the inhibitors and 2) DPP IV inhibitor treatment seems to inhibit L-cell secretion, probably because of negative feedback mechanisms. Furthermore, it is likely that the effect of the inhibitors on appetite, food intake and gastric emptying as seen with the analogues is weak or absent. This indicates that DPP IV inhibitors as monotherapy may not be sufficiently active in patients with poor and long-standing disease. In such patients GLP-1 analogues may be preferable.
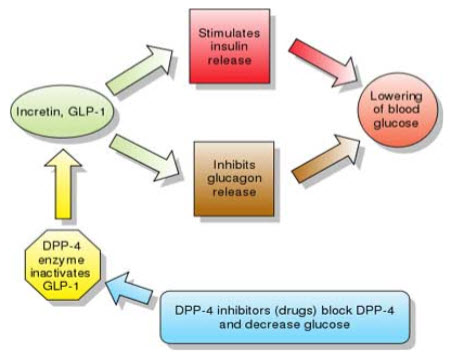
Figure 2: Mechanism of Action of Incretins
A number of DDP-IV inhibitors are under investigation for the treatment of type 2 diabetes in humans. Vildagliptin (Novartis) and sitagliptin (Merck) are currently the only two gliptins with clinical trial data looking at their use as monotherapy and combination therapy with existing oral hypoglycaemics.
Sitagliptin
In a 18-week study, Sitagliptin (100mg once-daily) significantly (p < 0.001) reduced HbA1c from baseline compared with placebo. Treatment with sitagliptin resulted in a significant (p < 0.001) reduction from baseline in FPG at Week 18 compared with placebo. Treatment with sitagliptin also resulted in significant (p < 0.001) reduction from baseline in 2-h PPG at Week 18, compared with placebo(18).
Vildagliptin
In a 12-week study, Vildagliptin (25mg b.i.d, n = 70) versus placebo (b.i.d., n = 28). A simultaneous improvement in β-cell function was also observed. Subjects who had higher baseline HbA1C levels showed greater response(19).
GLIPTINS: CURRENT STATUS
The advent of DPP-4 inhibitors symbolizes a key therapeutic advance in the management of T2DM. DPP-4 inhibitors are weight-neutral, not associated with fluid retention and do not cause any serious hypoglycemic events. These therapies have shown potential to improve glycemic control similar to existingOral Hypoglycaemin Agents, with prospective improvement in pancreatic β-cell function. In the new group of DPP-4 inhibitors, vildagliptin and sitagliptin has shown to be an effective and safe option for better glycemic control in a wide range of T2DM patients and promises to address limitations of current T2DM therapies. It also has the advantage of oral administration for patients who are either not candidates for or are resistant to injectable therapies.
Summary and Conclusion
With the growing numbers of patients suffering from diabetes the use of Gliptins or DPP-IV Inhibitors proved to be a novel approach. DPP-4 inhibitor has shown consistent and sustained efficacy and tolerability in clinical trials with reduced HbA1C, FPG, PPG levels and prandial glucagon secretion, improved β-cell function. They are generally well tolerated in patients, with a low incidence of gastrointestinal AEs, no weight gain and no reported events of hypoglycemia.
Acknowledgement:
The author would like to thank Ashok Kumar (Asst. Professor, PDM College of Pharmacy) for guiding a right path and for editorial support provided during preparation of manuscript of this article.
References
1. Unwin N, Whiting D, Gan D, Jacqmain O, Ghyoot G, editors. IDF Diabetes Atlas. 4th ed. Brussels: International Diabetes Federation, 2009
2. World Health Organization. Prevention of diabetes mellitus. Report of a WHO Study Group. Geneva: World Health Organization; 1994. No. 844.
3. Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001 Dec; 414(6865):782-787.
4. Reaven GM. 1998 The role of insulin resistance in human disease. Diabetes. 37:1595–1607.
5. Olefsky JM. 1989 Pathogenesis of non-insulin dependent diabetes (type 2). In: DeGroot LJ, Besser GM, Cahill JC, eds. Endocrinology, 2nd ed. Philadelphia: Saunders; 1369–1388
6. Ferner RE, Neil HAW. Sulfonylureas and hypoglycaemia. Br Med J (Clin Res Ed.) 1988; 296: 949-950
7. Seltzer HS. Drug-induced hypoglycaemia. A review of 1418 cases. Endocrinol Metab Clin North Am 1989; 18: 163-183.
8. Aronoff S, Rosenblatt S, Braithewaite S, et al. Pioglitazone hydrochloride monotherapy improves glycaemic control in the treatment of patients with type 2 diabetes: a 6-monthrandomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 2000; 23: 1605-1611.
9. Nauck M, Stockmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986; 29:46–52.
10. Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care 2003; 26:2929–2940.
11. Bloomgarden ZT. Gut hormones and related concepts. Diabetes Care 2006; 29:2319 2324.
12. Nauck MA, Kleine N, Orskov C. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993; 36:741–744.
13. Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes 1995; 44:1126–1131.
14. Holst JJ, Deacon CF. Glucagon-like peptide-1 mediates the therapeutic actions of DPP-4 inhibitors. Diabetologia 2005; 48:612–615.
15. Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 1999; 140:5356–5363.
16. Vilsboll T, Krarup T, Madsbad S, Holst JJ. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia 2002; 45:1111-19.
17. Vilsboll T, Knop FK, Krarup T. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide-regardless of etiology and phenotype. Journal of Clinical Endocrinology & Metabolism 2003; 88:4897-903.
18. Viswanathan Mohan, Wenying Yang, Ho-Young Son, Lei Xu, Liliane Noble, Ronald B. Langdon, John M. Amatruda, Peter P. Stein, Keith D. Kaufman. Efficacy and safety of sitagliptin in the treatment of patients with type 2 diabetes in China, India, and Korea. Diabetes and clinical practice 83 (2009) 106-116.
19. Pratley RE, et al. Horm Metab Res 2006;38(6):423-8.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE









