 About Authors:
About Authors:
Sofiya Verma*, Dipak V, Ashok A, Neha C, Jagruti R, Ashutosh. M
Shri ram institute of pharmacy
Jabalpur M.P
*sofiyavermamph@gmail.com
ABSTRACT:
The purpose of this research was to prepare and characterize theophylline microsphere of guar gum polymer for the application of nocturnal asthma. The microsphere were prepared by using emulsification method using sodium borate as a cross linking agent and coating was done by solvent evaporation method with the pH sensitive eudragit S-100 polymers. The prepared microsphere were white, free flowing and spherical in shape. The drug-loaded microsphere showed entrapment efficiency and drug release was extended upto 6 to 8 h. The infrared spectra, differential scanning calorimetry thermographs and XRD spectra all showed the stable character of both the drugs in the drug-loaded microspheres and revealed the absence of drug-polymer interactions. Scanning electron microscopy study revealed that the microspheres were spherical and porous in nature. The prepared theopylline microsphere has the potential for delaying the release of drug.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1611
INTRODUCTION
Delivery of colon mainly concerned with the delivery of drug into the lower GI tract, which occurs primarily in the large intestine (i.e., colon) [1]. Delayed systemic absorption of drugs via colonic delivery is desirable for chronotherapy of diseases such as asthma, hypertension, cardiac arrhythmias, arthritis or inflammation, which are affected by circadian biorhythms. These diseases are characterized by night-time or early morning onset. For treatment of these diseases, it is therefore highly desirable to have a delayed-release delivery system that can provide nocturnal release of a drug, which in turn may provide considerable relief to the patients while they are in resting [2].
Guar gum is a naturally occurring polysaccharides obtained from the seeds of cyamopsis tetragonolode having gelling property that retards release of the drug from the dosage form as well as it is susceptible to degradation in the colonic environment[3].The use of naturally occurring polysaccharides is attracting a lot of attention for drug targeting the colon since these polymers of monosaccharides are found in abundance, have wide availability are inexpensive and are available in a verity of a structures with varied properties. They can be easily modified chemically, biochemically, and are highly stable, safe, nontoxic, hydrophilic and gel forming and in addition, are biodegradable. These include naturally occurring polysaccharides obtained from plant (guar gum, inulin), animal (chitosan, chondrotin sulphate), algal (theophyllines) or microbial (dextran) origin. The polysaccrides can be broken down by the colonic microflora to simple polysaccharides. [4]
[adsense:468x15:2204050025]
MATERIALS AND METHODS
Materials
The drug (theophylline) was found as a gift sample from Cipla Limited, Mumbai, India. Guar gum, sodium borate, castor oil, span 60 and tween 80 were purchased from Central Drug House Pvt. Ltd. All the other reagent used during experiment were of analytical reagent grade. Double distilled water was used during the whole experiment.
Methods
Guar gum microspheres were prepared by using the method reported by Chaurasia et al. [5] with slight modification. Guar gum dispersion was prepared by mixing of guar gum with tween 80 (0.2% w/w) followed by the addition of distilled water in which THEO was previously dissolved and allowed to swell for 2 h. This aqueous phase was dispersed in castor oil (100ml) containing span 60 (5% w/w) and maintained at 60 °C. This dispersion was stirred employing a mechanical agitation with propeller stirrer (Remi, Mumbai, India) at 2000 rpm. After 10 min., sodium borate (5 ml) and stirring was continued for 2 h at constant speed. The microspheres were centrifuged (CFC-FREE, C-24, Cooling centrifuge, REMI, India), washed with isopropyl alcohol to remove the oil and dried under vacuum oven (Dolphin, India).
Coating of Eudragit S-100 (es) on prepared microsphere
Encapsulation of Guar Gum Microspheres: - Guar gum microspheres (core) were coated with Eudragit polymer by oil-in-oil solvent evaporation method. Coating solution (5%) was prepared by dissolving 1:1 mixture of Eudragit S-100 (ES) in 10 ml of organic solvent (acetone: ethanol, 1:1). Guar gum microspheres (50 mg) were dispersed in this organic phase and poured in 70 ml light liquid paraffin containing 1% w/v Span 85. The system was stirred at 1000 rpm speed using a mechanical stirrer at room temperature for 3 h to allow the evaporation of solvent. Finally, the coated microspheres were collected by centrifugation, washed with n-hexane; freeze dried overnight (Heto Drywinner, Denmark) and kept in airtight container for further studies.
Optimization
Various formulation variables e.g polymer concentration, drug concentration, emulsifier concentration, concentration of cross-linking agent and process variable viz. stirring speed, which would affect the preparation and properties of microspheres were identified and studied (table 1).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table 1: Effect of varying parameteron particle size, swelling index and drug entrapment efficiency of microspheres
|
Formulation |
Variables |
Particle size* |
Swelling index* |
Entrapment efficiency*/% drug release* |
|
Polymer concentration (mg) |
||||
|
P-1 |
1 |
150.29±2.3 |
1.38 ±1.35 |
62.2 ±0.33 |
|
P-2 |
2 |
152.57±3.3 |
1.26 ±1.25 |
80.0 ±0.2 |
|
P-3 |
4 |
168.8 ±2.3 |
1. 43 ±1.21 |
75.4 ±1.73 |
|
P-4 |
5 |
169.7 ± 3.5 |
1.61±0.21 |
72.8 ±4.19 |
|
Drug concentration (mg) |
||||
|
E–1 |
50 |
166.57±3.24 |
- |
75.8±4.19 |
|
E–2 |
100 |
167.57±2.95 |
- |
79.0±0.21 |
|
E–3 |
150 |
169.67±1.33 |
- |
76.4±2.39 |
|
E–4 |
200 |
179.77±3.87 |
- |
78.5±1.72 |
|
Emulsifier concentration (%)span 60 |
||||
|
B–1 |
1.5% |
179.08±2.23 |
- |
60.52 ±0.15 |
|
B–2 |
2.0% |
164.4 ±2.38 |
- |
63.71 ±0.95 |
|
B–3 |
3.0 % |
160.16 ±3.58 |
- |
72.8 ±2.19 |
|
B–4 |
5.0% |
154.14 ±3.51 |
- |
75.3 ± 1.37 |
|
Cross-linking agent (ml) |
||||
|
C–1 |
1 |
170.17±1.58 |
- |
67.2 ±0.2 |
|
C–2 |
2 |
168.41 ±3.95 |
- |
69.3 ±1.01 |
|
C–3 |
3 |
171.72 ±1.53 |
- |
72.15 ±4.55 |
|
C–4 |
5 |
169.41 ±2.86 |
- |
75.70 ±0.20 |
|
Stirring speed (rpm) |
||||
|
D–1 |
500 |
172.11±0.34 |
- |
64.1±0.12 |
|
D–2 |
1000 |
168.58±4.49 |
- |
65.5±0.20 |
|
D–3 |
1500 |
165.91±3.38 |
- |
76.2±1.09 |
|
D–4 |
2000 |
160.37±0.33 |
- |
78.3±0.10 |
|
Eudragit S-100 |
||||
|
E–1 |
5 |
172.67±1.33 |
1.22±0.05 |
80.0 |
|
E–2 |
10 |
186.57±3.24 |
0.25±0.09 |
74.4 |
|
E–3 |
15 |
197.57±2.95 |
1.11±0.18 |
65.8 |
*Values represent mean ±SD (n=3)
1 Particle size
Particle size and size distribution of different microsphere formulations were measured using an optical microscope, and the mean particle size was calculated by measuring 200 particles with the help of a calibrated ocular micrometer. The average particle size was expressed as the volume mean diameter in micrometers. [6, 7].
2 Shape and surface Morphology
The morphology and appearance of microparticles were examined by scanning electron microscopy (SEM). The prepared microspheres were freeze dried at -30oC for 48 h and coated with gold palladium under an argon atmosphere for 150 s to achieve a 20 nm film (Sputter coater, SCD 004, BAL-TEC, Balzers, Furstentum, Lienchestein). The coated samples were then examined with a scanning electron microscope (Jeol JSM-1600, Tokyo, Japan). ). The scanned and photomicrographs were taken, which as shown in Fig. 4.13 (a) and (b).
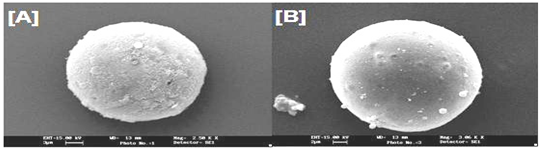
Figure 1: Scanning electron micrographs of (A) cross-linked Guar gum microsphere and (B) Eudragit S-100 coated cross linked Guar gum microsphere.
3. Entrapment efficiency
Entrapment efficiency was determined by using the method reported by Chaurasia et al.[5] .An accurately weighed quantity of guar gum microspheres (equivalent to 50 mg of THEO) was incubated in 10 ml of PBS (pH 7.4). The sample was ultrasonicated for 3 consecutive periods of 5 min. each, with a resting period of 5 min. each and kept for 48 h for complete extraction of theophylline. The solution was centrifuged and supernatant was assayed for THEO spectrophotometrically (UV-1800 Spectrophotometer, Shimadzu, Japan) at 271.5 nm. Each determination was made in triplicate. The drug entrapment efficiency was calculated using the following formula.
Entrapment Efficiency =
Estimated % Drug Content
---------------------------- X 100 ...5.1
Theoretical % Drug Content
4. Swell ability
A known weight (100 mg) of guar gum microspheres and Eudragit coated guar gum microspheres were placed in enzyme free simulated intestinal fluid (SIF) and allowed to swell up to constant weight at 37 ± 0.5 ºC in the dissolution apparatus (USP XXIII Model DT-06, Erweka, Germany). The microspheres were periodically removed, blotted with filter paper and their change in weight was measured until attainment of equilibrium. The swellability is reported in the Table 1.
5. Differential scanning calorimeter.
In order to determine the physical state of drug, i.e. amorphous or crystalline, before and after final formulation and to evaluate any possible drug–polymer, drug–other components interaction [9]. differential scanning calorimeter (DSC) of, theophylline, drug polymer mixture (1:1), blank and drug-loaded theophylline microspheres were carried out by heating the sample from 30°C to 350°.C at the heating rate of 10°C/min. in a nitrogen environment (nitrogen gas flow rate of 20ml/ min).
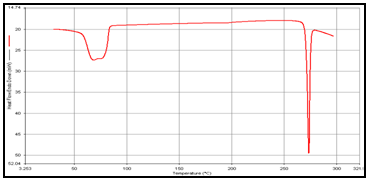
Figure 2: DSC of (a) Theophylline,
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
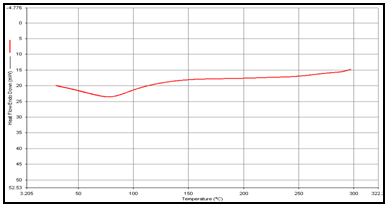
(b) Guargum
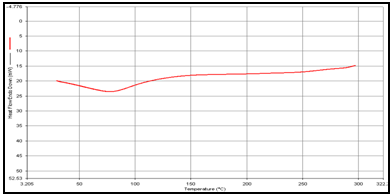
(c) Mixture of Theophylline and Guargum,
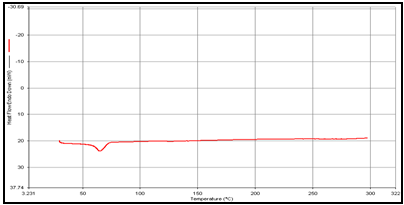
(d) Uncoated guargum microsphere
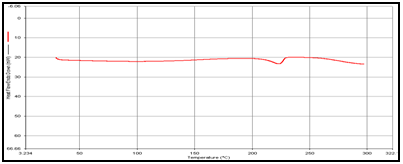
(e) Coated guargum microsphere
6 .X-ray diffraction (XRD)
X-ray diffraction (XRD) patterns of THEO, guargum, physical mixture (1:1) and optimized blank and loaded formulations were measured by X–ray diffrectometer at 5–50°. Crystallinity of powder, vehicle and the formulations were determined at 2θ degree min–1. Scanning rate, 40 KV and 30 mA with Rigaku generator.
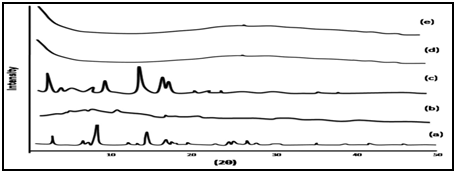
Figure 3: XRD of (a) Theophylline, (b) Guargum, (c) Mixture of Theophylline and Guargum, (d) Uncoated guargum microspheres, (e) Coated guargum microspheres.
7. In vitro drug release studies
The release of thophylline from guar gum microspheres and Eudragit coated guar gum microspheres was investigated using rotating paddle dissolution apparatus at rotation speed of 100 rpm. The system was thermostated at 37 oC. Drug release was measured from accurately weighed amount of microspheres, equivalent to 100 mg of theophyline, added to 500 ml of dissolution medium. For microspheres, simulation of gastrointestinal transit conditions was achieved by pH progression medium. The pH of the dissolution medium was kept 1.2 for 2 h with 0.1 N HCl. Then 1.7 g of KH2PO4 and 2.225 g of Na2HPO4 .2H2O were added adjusting the pH 4.5 with 1.0 M NaOH and release rate study was continued for further 2 h. After 4 h the pH of dissolution medium was adjusted to 7.0 and maintained up to end of study. The final volume in all cases was 500 ml. The samples were withdrawn from dissolution medium at various time intervals using a pipette fitted with a micro filter and analyzed spectrophotometrically at 271.5nm. All dissolution studies were performed in triplicate.
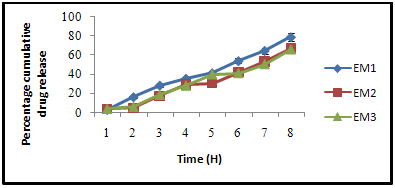
Figure 4: Effect of varying Eudragit S-100 agent on dissolution rate of guargum loaded theophylline microsphere.
8. In vitro drug release study in the presence of rat caecal content
Rat caecal content was prepared by the method, reported by Mooter et al. and Paharia et al. [8,9]Albino rats (Sprague-Dawley strains) of uniform body weight (180-200g) with no prior drug treatment were used for all the present in vivo studies, were weighed, maintained on normal diet and administered 1 ml of 2% dispersion of guar gum/EL/ES in water and this treatment was continued for 7 days to induce enzymes acting on polymers. Thirty minutes before starting the study, the rat was sacrificed, the abdomen was opened, caecal were traced, legated at both end dissected and immediately transferred into pH 6.8 (PBS), which was previously bubbled with CO2. The caecal bag was opened; contents were weighed, homogenized, and then suspended in PBS (pH 7.4) to give the desired concentration (2 %) of caecal content, which was used as simulated colonic fluid. The suspension was filtered through cotton wool and ultrasonicated for 10 min in an ice bath at 40% voltage frequency using a probe sonicator (Frontline Electronics, India) at 4°C to disrupt the bacterial cells. After sonication, the mixture was centrifuged (Remi, India) at 2000 rpm for 20 min. A weighed amount of microspheres was placed in 200 ml of dissolution media (PBS, pH 7.4) containing 2% w/v rat caecal content. The experiment was carried out with continuous CO2 supply into the dissolution medium. At different time intervals, the samples were withdrawn and replaced with fresh PBS. The experiment was continued up to 24 h. The withdrawnsamples were pipetted into a series of 10 ml volumetric flasks and volumes were made up to the mark with PBS and centrifuged. The supernatant was filtered through 0.45μm membrane filter (Millipore) and the filtrate analyzed for THEO content at 271.5 nm using UV spectrophotometer. All the experiments were performed in triplicate.
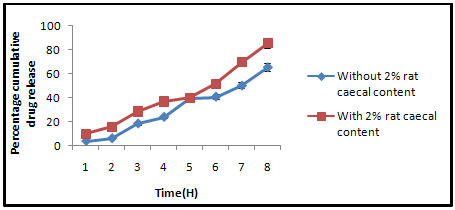
Figure 5: Percentage of cumulative drug release in PBS (7.4) with and without rat caecal content.
RESULTS AND DISCUSSION
Particle size of the Guargum microspheres increased from 150.29 ± 2.3 µm to 169.7 ± 3.5 µm with increasing polymer concentration from 200 mg to 500 mg. A higher concentration of polymer produced a more viscous dispersion, which formed larger droplets and consequently larger microspheres as reported by Pongpaibul et al. The swellability is also increases with increase in polymer concentration from 1.38 ± 1.3 to 1.61±0.21. The drug entrapment efficiency increased from 62.2 ± 0.33 to 80.0 ± 0.2 as the concentration of polymer increases beyond which on increase the drug entrapment efficiency remained unchanged.
On varying the drug concentration, the particle size of Guargum microspheres almost remain unchanged which may be due to constant polymer concentration. The drug entrapment efficiency increase with an increase in drug concentration. Due to greater loading of the drug in microsphere but after 100 mg the drug entrapment efficiency remained constant. which may be due to no free space available in microsphere to accommodate greater quantity of drug the results shown that the minute change in the size of microsphere as on increasing the drug concentration but the percent drug entrapment efficiency enhanced upto 20%, the drug resulting in entrapment efficiency of the drug increases.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
The particle size of Guargum microsphere decreased from 179.08±2.23 to 154.14±3.51.with increasing emulsifier concentration. This was due to stabilization of the emulsion droplets avoiding their coalescence as reduction of interfacial tension between guargum and oil droplets, occurs resulting in, smaller size microspheres similar results are reported by by Maia et al.The drug entrapment efficiency increased from 75.21 ± 4.19 to 78.5 ± 1.72
The drug entrapment efficiency varied from70.2±0.22 to 85.15±4.55 (table 1). The particle size of theophylline microspheres showed little difference from 313.14±1.58 to 382.4±2.86 μm when increasing concentration of sodium borate from 1 to 5ml to 1 to 5ml and entrapment efficiency varied from70.2±0.22 to 85.15±4.55 (table 1). And similar result was found as reported by Esposito et al.
The particle size of Guargum microspheres decreased from 172.11 ± 0.34 µm to 160.37 ± 0.33 µm with increasing stirring rate from 500 rpm to 2000 rpm. Results revealed that the particle size of microspheres was controlled by stirring rate. This may be due to the smaller emulsion droplets produced by a higher stirring speed, which provided more energy to disperse the oil phase in water, but higher agitation speeds upto 1250 rpm resulted in irregularly shaped microspheres. Similar result is reported by Jain et al.The drug entrapment efficiency increased from 64.11± 0.12 to 75.70 ± 0.20(table 5.5).The drug entrapment efficiency increases due to decrease in surface area on reduction of the size of microspheres. Similar results are reported by Jain et al.
The prepared Guargum microspheres were coated with Eudragit S-100 by method repoted by choursia et al.Eudragit coating was done to govern the drug release of microsphere in large intestine. Varying concentration of Eudragit S-100 was used to evaluate the rate of drug release. (table 5.6).It was observed that rate of drug release was found to decreases with an increase in coating concentration. This may be attributed due to greater viscosity of the coating material which retards the rate of drug release from guargum microsphere.
SEM was used to investigate the morphology as well as particle size of microspheres. As showed in Figure 5.9 (a) and (b), microspheres displayed a spherical shape with a smooth surface and no aggregation was observed.
DSC studies were performed to investigate the physical state of the drug in the microspheres, because this aspect could influence the in-vitro release of the drug from the systems. Different mixture of drug/polymer may co-exist in the polymeric carriers, such as: (i) amorphous drug in either an amorphous or a crystalline polymer and (ii) crystalline drug in either an amorphous or a crystalline polymer. Moreover, a drug may be present either as a solid solution or solid dispersion in an amorphous or crystalline polymer Figure 5.10. show the DSC thermograms of pure (a)Theophylline, (b) Guargum, (c) physical mixture of drug and polymer (1:1), (d) uncoated guargum misrosphere(e) coated guargum microspheres,respectively. Pure Theophylline showed an endothermic melting peak at 70.0020C. Guargum showed an endothermic peak at 74.9860C. The endothermic peak of the physical mixture of guargum and theophylline was observed at 69.9950C so the peakwere similar to the melting point of guargum and theophylline. Theophylline melting peak totally disappeared in the thermogram of loaded microspheres, evidencing the absence of crystalline drug in the microsphere samples, at least at the particle surface level. Therefore, it could be concluded that theophylline in the microspheres was in amorphous phase of a molecular dispersion or a solid solution state in the polymer matrix after the production.
XRD Studies of the formulation of microspheres was verified by characterization of 2θ (degree) using XRD. Figure 5.11 shows the XRD pattern for the theophylline, guar gum, physical mixture, uncoated and coated preparation. As shown in this figure, the diffraction peaks of drug which showed its crystalline nature. Pure guar gum did not showed any peak; indicating its amorphous nature. Similarly the microspheres prepared with guar gum with and without drug also did not showed any peak confirming that polymer shield drug and show good entrapment. Figure.5.11 also represents the XRD pattern for blank and loaded preparation indicating the stability of the preparation in the presence of guar gum
In-vitrodrug release study of all microspheres was performed in simulated gastrointestinal fluid medium of different pH. (table 5.7).The rate of drug release was found to decrease with an increase in polymer concentration. This decrease in the rate and extent of release with relative increase in the polymer concentration in microspheres can be attributed to the increase in the density of the polymer matrix with increased polymer concentration resulting in slower rate of diffusion through the polymer matrix as similar results are reported by castelli et al.
The invitro drug release rate from varying concentration of Eudragit coated Guargum microspheres was also studied. It was observed that with an increases in coating material composition the rate of drug release decreases. The release pattern of Eudragit coated Guargum microsphere can be understood by the protective nature of Eudragit coating delaying the drug release in upper GI tract as similar results are reported by Rai et al.
The invitro drug releae of Eudragit coated Guargum microsphere was done in the presence of 2% w/v rat cecal content,using a USP dissolution test apparatus. The presence of rat cecal content in the dissolution medium increased the rate of drug release from the Guargum microspheres compared with the control. In-vitro drug release in simulated colonic fluid without rat cecal material was 65.68 ± 1.43 %, but drug release in simulated colonic fluid with 2% rat cecal content after 8 hrs was 85.42 ± 1.86 %. The rat cecal material in the dissolution medium had increased the drug release from Guargum miscrospheres, which may be attributed to Guargum degradation by various anaerobic bacteria producing enzymes (mainly Amylase) present in the caecum simiar results are reported by Jain et al. Hence, the drug release in the simulated colonic fluid with cecal content may be a result of the combined effects of diffusion and erosion.
The results of our study clearly indicate that Guargum microspheres have potential in delivery of theophylline to the colonic region as an alternative to the conventional dosage form. Various formulation as well as process variables affect the size, shape and drug release pattern of microspheres. Guargum is a biocompatible polymer; we expect it to cause no harmful effects if used for prolonged periods. Guargum microspheres showed good entrapment efficiency and produced spherical particles. Guargum microspheres bearing Theophylline maintain their integrity in the hostile environment of stomach and small intestine and this system showed pH dependent drug release in GI tract. In the upper part of GI tract Guargum microspheres show small amount of drug release. Hence, it can be concluded that Guargum microspheres can be utilized for the site specific delivery of the drug to the colon. We can conclude that Guargum microspheres of Theophylline showed extended drug release profile in pH progression medium. Therefore, designed drug delivery system can be effectively used for the treatment of pain and inflammation.
CONCLUSIONS
The present study demonstrates successfully chronopharmaceutics based colonic drug delivery system for nocturnal asthma. The experimental results demonstrated that Eudragit coated guar gum microspheres have potential to be used as a drug carrier for an effective colon targeted delivery system.
ACKNOWLEDGEMENT
The authors are thankful to Principal and Head of department of SRIT College of Pharmacy, Jabalpur for providing necessary laboratory facilities to carryout this work with great ease and precision and also thanks to Vikas sir and Aditya sir for, giving pivotal guidness.
REFERENCE
1. Nirav Patel, Jayvadan Patel, Tejal Gandhi, Tejal Soni, Shreeraj Shah, Novel Pharmaceutical Approaches for Colon-Specific Drug Delivery: An Overview Review Article Journal of Pharmacy Research 2008;1:1.
2. Editor(s), compiler(s) as author: Singh BN, Kim, KH.,Swarbrick J, Boylan JC, (editors.), Encyclopedia of Pharmaceutical Technology, New York, Marcel Dekker, I n c. (2002).
3. Kosaraju S. L.Crit. Rev. Food Sci. Nutr., 2005; 45: 251-258
4. Philip Anil K., Philip Betty,Colon Targeted Drug Delivery Systems: A Review on Primary and Novel Approaches Oman Medical Journal 2010; 25: 2.
5. Chourasia Mk, Jain S.k. Pharmaceutical Approaches to Colon targeted drug Delivery System J Pharma Pharmacet Sci 2003;61: 33-44.
6. Ashord M, Fell JT, Attwood D, Sharma H, Woodhead P. An evaluation of pectin as a carrier for drug targeting to the colon. J Control Rel 1993; 26:213-220.
7. Editor(s), compiler(s) as author: Krishnaiah YSR, Styanarayana S. (editors) Colon specific drug delivery systems. CBS Publishers and Distributors: 2000.
8. Mooter GV, Samyn C, Kinget R. In vitro evaluation of a colon specific drug delivery system: An absorption study of theophylline from capsules coated with azo polymers in rats. Pharm Res 1995; 12(2):244-247.
9. Paharia A., Yadav A. K., Rai G.. Jain S. K., Pancholi S., Agrawal G. P., AAPS PharmSciTech.2007; 8:E1-E7.
10. Pongpaibul Y, Price JC, Whitworth CW. Preparation and evaluation of controlled release indomethacin microspheres. Drug Dev Ind Pharm 1984; 410:1597-616
11. Maia JL, Santana MH, Re MI. The effect of some processing conditions on the characteristics of biodegradable microspheres obtained by an emulsion solvent evaporation process. Braz J Chem Eng 2004; 21:1-12
12. Esposito E, Cortesi R, Luca G, Nastruzzi C. Pectin-Based Microspheres: A Preformulatory Study. Ann NY Acad Sci 2001; 944:160-7
13. Jain SK. Chourasia MK, Potential of guar gum microspheres for target specific drug release to colon. J Drug Target. 2004; 12: 435Y442.
14. Chourasia MK, Jain SK. Design and development of multiparticulate system for targeted drug delivery to colon. Drug Deliv. 2004;11:201Y207
15. Castelli et al., Eudragit as controlled release system for anti-inflammetry drugs A comparision between DSC and dialysis experiments; Thermochimica Acta. 2003; 400:227-234.
16. Rai G., Jain S. K., Agrawal S., Bhadra S., Pancholi S. S., Agrawal G. P., Pharmazie.2005 2:131-134.
17. Jain SK, Rai G, Saraf DK, Agrawal GP. The preparation and evaluation of albendazole microspheres for colonic delivery. Pharmaceutical Technology. 2004; 66-71.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









