 About Authors:
About Authors:
DILIP KUMAR
Department of Pharmaceutics,
Rajiv Academy for Pharmacy, Mathura-286001, Uttar Pradesh, India
kumardilip.pharma@gmail.com
ABSTRACT:
The aim of the present study was to prepare and evaluate buccal tablet comprising a drug-containing buccoadhesive layer and a drug-free backing layer, by the direct compression method. The mucoadhesive layer was composed of a mixture of drug, hydroxypropylmethylcellulose (HPMC K4M), Carbopol 934P (CP), Sodium carboxy methyl cellulose (NaCMC), and the backing layer was made of ethyl cellulose. Enalapril maleate is an ace -inhibitor was formulated onto buccoadhesive tablets to overcome the limitations in the currently available dosage forms and routes of administration which in sequence will increase patient’s compliance. Formulations (F1-F9) were developed and subjected to various evaluation parameters. All tablets were acceptable with regard to thickness, weight variation, hardness, and drug content. Maximum bioadhesive force was observed in tablets formulated using CP-NaCMC as a bioadhesive polymer (F4-F6). Formulation F6 showed maximum permeation of 91.98 % ± 0.58 in 8hr. Formulation F3 showed maximum swelling index of 2.99 ± 0.01 after 8hr. The mucoadhesive force and residence time of the optimized batch F6 are 0.14 N ± 0.01 and 9.10 hrs ± 1.15 respectively. The results indicate that suitable buccoadhesive tablets with desired properties could be prepared.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-2046
INTRODUCTION:
The buccal mucosa offers excellent opportunities for the delivery of both local and systemically active drugs1, 2. Bioadhesion may be defined as the state in which two materials, at least one of which is of biological nature, are held together for extended periods of time by interfacial forces3. Amongst the various routes of drug delivery, the oral route is most preferred by patient and the clinician alike because of the significant attention to their presystemic metabolism or any instability in the acidic environment associated with the oral environment. Consequently, other absorptive mucosa, are considered as potential site for drug administration, rich blood supply, lower enzymatic activity of saliva, better patient acceptance are some other prominent meritorious visage of buccoadhesive systems 4,5.
Enalapril maleate is a low dose angiotensin converting enzyme (ACE inhibitor) used to treat hypertension and heart failure. It inhibits angiotensin – converting enzyme, which is involved in the conversion of angiotensin-I to angiotensin-II stimulate the synthesis and secretion of aldosterone and blood pressure via a potent direct vasoconstrictor effect6. Due to the extensive first pass metabolism it shows low oral bioavailability of 40 %, 7, 8 has the log P value of 2.45 and pKa of 3 which makes it a suitable candidate for oral mucosal drug delivery system9. The maximum plasma concentration of enalapril maleate is not detected above 10 ng after 4 hrs, from a single oral dose of 10 mg. 10, 11 buccal films of enalapril maleate was not capable for maintaining the salivary concentration of drugs for a prolonged period due to its less residence time because of inefficient mucoadhesive property of different polymeric concentration and therefore, it washed out with saliva12. Hence, in the present work, an attempt was made to formulate buccoadhesive buccal tablets of enalapril maleate as layered structure to provide a unidirectional controlled delivery of drug release that has potential & avoid extensive hepatic fist pass metabolism. Some advantages of the buccal tablets were including: avoidance of first pass effect, protect drugs that are unstable in acidic environment of the stomach and the dosage form can easily removed.
Rational blends of carbopol 934P, HPMC K4M (hydroxypropylmethylcellulose), NaCMC (sodiumcarboxymethylcellulose), and mannitol were chosen as the polymers to formulate buccal bioadhesive tablet of the drug.
[adsense:468x15:2204050025]
MATERIALS AND METHODS
The drug enalapril maleate was received as a gift sample from cadila pharma. Ltd. (Ahmadabad, India) carbopol 934P, HPMC K4M and NaCMC was provided by Central Drug house (P) Ltd. (New Delhi, India), Mannitol and ethyl cellulose was procured by S.D. fine Chemicals Ltd. (Mumbai, India).
Preparation of buccoadhesive tablets of Enalapril maleate
Direct compression method has been employed to prepare buccal tablets of enalapril maleate, carbopol 934P, HPMC K4M, NaCMC, and mannitol as polymers. All the ingredients including drug, polymers and excipients were weighed accurately. The drug was thoroughly mixed with the mannitol and all ingredients except lubricants were mixed in order of ascending order and blended for 10 min in an inflated polyethylene pouch. After uniform mixing of ingredients, lubricant was added and again mixed for 2 min. The prepared blend (100 mg) of each formulation was compressed, on single tablet punching machine to form single layered flat faced tablet of 9 mm diameter. Then 50 mg of ethyl cellulose powder was added in the die and final punching was done to get layered tablets (Table 1).
Table 1. Composition of formulations
|
Formulation Codes |
Drug /tablet (mg) |
HPMC K4M (mg) |
NaCMC (mg) |
CP 934P (mg) |
Mannitol (mg) |
Ethyl cellulose (mg) |
|
F1 |
10 |
20 |
20 |
- |
50 |
50 |
|
F2 |
10 |
20 |
40 |
- |
30 |
50 |
|
F3 |
10 |
20 |
60 |
- |
10 |
50 |
|
F4 |
10 |
- |
20 |
20 |
50 |
50 |
|
F5 |
10 |
- |
20 |
40 |
30 |
50 |
|
F6 |
10 |
- |
20 |
60 |
10 |
50 |
|
F7 |
10 |
20 |
- |
20 |
50 |
50 |
|
F8 |
10 |
40 |
- |
20 |
30 |
50 |
|
F9 |
10 |
60 |
- |
20 |
10 |
50 |
EVALUATION OF BUCCAL TABLETS
Weight Variation and Hardness Test
The weight variation test was done by weighing 20 tablets individually, calculating the average weight and comparing the individual tablet weights to the average. From this, percentage weight difference was calculated and then checked for USP specifications. The tablets were tested for hardness by Monsanto Hardness Tester (Hicon, Grover Enterprise).
Swelling study
Three tablets were tested for each formulation. The tablet were weighed individually (W1) and placed separately in petridishes containing 5 ml of phosphate buffer, pH 6.8. At regular intervals of 1, 2, 4, and 8 hrs the tablets were removed from the petridishes and excess surface buffer was removed carefully using the filter paper. The swollen tablets were then reweighed (W2) and swelling index (SI) was calculated using eq. 1. The experiments were carried out in triplicate and average values are reported.
SI (%) = (W2-W1) X 100 ..............eq. 1
W1
Drug content
Drug content was determined by homogenization of three tablets in 100 ml phosphate buffer (pH 6.8 and pH 7.4) separately, filtered through 0.45μ filter diluted suitably and analyzed spectrophotometrically at 207 nm and 213 nm respectively (UV spectrophotometer, Shimadzu Pharma spec 1700, Tokyo, Japan). The experiments were carried out in triplicate and average values are reported.
Ex-vivomucoadhesion time
The ex-vivo mucoadhesion time was evaluated after application of the tablet onto freshly cut porcine buccal mucosa tissue, procured from the local slaughter house and placed in buffer solution. The fresh porcine buccal mucosawas fixed in the inner side of the beaker, above 2.5 cmfrom the bottom, with cyanoacrylate glue. One side ofeach tablet was wetted with one drop of phosphatebuffer pH 6.8 and adhered to the porcine buccal mucosa byapplying a light force with a fingertip for 30 sec. The beakerwas filled with 500 ml of phosphate buffer pH 6.8and was kept at 37±1°C. After 2 min, a 50 rpm stirring ratewas applied to simulate the buccal cavity environment,and tablet adhesion was monitored up to 10 hr. The timerequired for the tablet to detach from the porcine buccalmucosa was recorded as the mucoadhesion time.
Ex- vivo mucoadhesion force
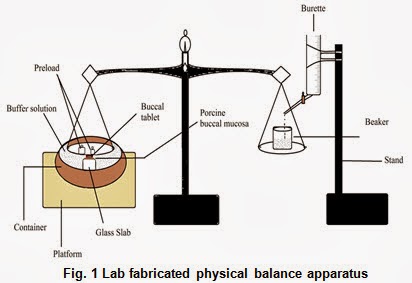
Fabricated modify physical balance apparatus (Fig. 1) was used for measuring the ex-vivo mucoadhesive force of the tablets.The porcine cheek pouch, excised and washed was fixed to the movable platform that is glass slab. The backing layer site of buccoadhesive tablet was fixed to the lower side of the pan containing the preload. The exposed tablet surface was moistened with one ml of phosphate buffer pH 6.8 for thirty seconds for initial hydration and swelling. The platform was then raised upward until the hydrated tablet was brought into the contact with the mucosal surface. A preload were placed over the pan for 5 min as initial pressure to establish adhesion bonding between tablet and porcine buccal mucosa. After completion of the preload time, preload was removed from the beaker, and water was then added into the beaker from the burette at a constant rate drop by drop. The addition of water was stopped when tablet was detached from porcine buccal mucosa. The weight of water required to detach tablet from buccal mucosa was noted as mucoadhesive strength, and these experiments were repeated with fresh mucosa in an identical manner (n=3).Mucoadhesive force was than calculated according to the following equation:
Force of adhesion (N) = [Mucoadhesive strength (g)] ×9.8 ...... eq. 2
1000
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Ex- vivo diffusion study
The ex-vivo drug diffusion study of enalapril maleate through the porcine buccal mucosal membrane (obtained from local slaughter house and used within 2 hr of slaughter), by using franz diffusion cell. The tissue was stored in phosphate buffered saline pH 7.4 solution upon collection. The epithelium was separated from the underlying connective tissues with surgical scissors and clamped in between the donor and the receiver chambers of the diffusion cell for permeation studies. The donor compartment was filled with three ml. of phosphate buffer (pH 6.8) while the receptor compartment contained 15 ml of phosphate buffer (pH 7.4). The upper compartment simulated the buccal cavity pH, and the lower compartment simulated the physiological pH of 7.4 for blood. The buccoadhesive side of tablet was placed towards mucosal surface of the porcine buccal mucosa in the donor compartment and two ml. aliquots were removed at 0, 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 hrs from the receptor compartment while the solution was being stirred continuously using magnetic stirrer. Two millilitre of fresh medium was replaced in the receptor compartment to maintain sink condition each time. The experiment was carried out at 37±0.5°C. The amount of drug permeated was analyzed at 213 nm. The graph of % drug permeated versus time was plotted. The experiments were performed in triplicate, and average values were reported (Table 3). The permeability coefficient and flux values were calculated using following equations,
Permeability coefficient (P) =Slope × volume of donor compartment (VD) .... eq. 3
Surface area of tissue used (S)
Flux (J) = Permeability coefficient (P) × concentration of donor compartment (CP)
........eq. 4
Drug release from backing layer
For determination of drug release from the backing layer, Franz diffusion cell was used. A buccal tablet was placed between donor and receptor compartment. The complete unit was maintained at 37±0.5°C, donor compartment (3 ml) was filled with phosphate buffer pH 6.8 and receptor compartment (15 ml) contained phosphate buffer pH 7.4 with continuous stirring. At predetermined intervals of 1, 2, 3, 4, 5, 6, 7, and 8 hr 1 ml. sample was removed from donor compartment and at 213 nm to check release of drug from the backing layer of the tablet.
Stability studies in simulated saliva
The stability of developed tablet was tested in simulatedsaliva pH 6.8 (sodium chloride 4.5 g, potassium chloride 0.3 g, sodium sulfate 0.3 g, ammonium acetate 0.4 g, urea 0.2 g, lactic acid 3 g and distilled water up to 1,000 ml, adjusting pH of solution to 6.8 by 1 M NaOH solution). The tablet was placed in a petri-dish containing 5 ml. of eight hrs simulatedsaliva and placed in a temperature-controlled oven at 37± 2°C. At regular time intervals of 0, 1, 2, 3, 4, 5, 6, 7, and 8 hr, the tablets were examined for change in colour, thickness and for Enalapril maleate. The experiments were repeated in triplicate and average values are reported.
DIFFERENTIAL SCANNING CALORIMETRY (DSC)
Pure drug (Enalapril maleate), polymers (Carbopol 934P, HPMC K4M and NaCMC) and formulation [F6] were subjected to thermal analysisusing differential scanning calorimetry (DSC Q200 V24.4 Build 116, TA Instrument, USA). Ten milligram of sample was accurately weighed into aluminium pans and then hermetically sealed with aluminium lids. Thermograms of the samples were obtained at a scanning rate of 100C/min over a temperature range of 20–450 0C.
HISTOPATHOLOGICAL EVALUATION OF BUCCAL MUCOSA
Histopathological evaluation of tissue (control) incubated in phosphate buffer saline solution pH 6.8 was compared with that treated with buccal tablet for eight hr. The tissue was fixed with 10 % formalin, routinely processed and embedded in paraffin wax. Paraffin sections were cut on glass slides by microtome and stained with hematoxylin and eosin. A pathologist blinded to the study to detect any damage to tissue. The tissue sections examined on light microscope at 10X magnification (Fig. 5).
RESULT AND DISCUSSION
Preparation of buccoadhesive tablet of Enalapril maleate
The bioadhesive polymers such as carbopol 934P, hydroxypropylmethylcellulose and sodium carboxy methyl cellulose were suitable for the preparation of buccoadhesive tablets because by the uptake of water they can easily stick to the oral mucosa and control the drug release 13. They resist the salivary secretion, tongue movement and swallowing for a significant period of time and an antihypertensive enalapril maleate was suitable for buccal tablet in the form of layered tablet. The results of the physicochemical parameters of the enalapril maleate buccal tablets shown in Table 2. Mannitol was used as channelling agent.
EVALUATION OF BUCCAL TABLETS
Measurement of characteristics of EM tablets
After the preparation of buccal tablets each tablets was evaluated for various physical characteristics. The shape of the tablets of all formulations was circular. The tablets weight varied between 149.25 ± 0.35 to 150.50 ±0.70 mg and the friability of all the formulations ranged between 0.05± 0.07 % to 0.40 ± 0.14 %. There is slightly variation in the friability of all the formulations due to the different polymers with different polymeric concentration. The maximum friability showed by the formulation F3 (0.40±0.14) having the combination of HPMC and NaCMC. In F3 formulation, concentration of NaCMC will increases which having the hygroscopic propertyand hygroscopic material and having the ability to absorb a large quantity (>50 %) of water under high humidity conditions. In tablets preparation the hygroscopicity is associated with decrease in tablet hardness24 and the friability increases. In formulations F4-F6 and F7-F9 having the combination of NaCMC-CP934P and HPMC-CP934P the friability will decreases in comparison to F1-F3 formulation. In these formulations used polymers are bioadhesive in nature due to which the hardness will increase and the friability decreases. The pharmacotechnical parameters of enalapril maleate tablets are shown in Table 2. Since NaCMC has good compressibility for tableting. NaCMC was used for comparison in the study. The lowest value of the hardness in the F2 formulation which contains the sufficient amount of the NaCMC and HPMC K4M and formulation F7 having the value 7.33 kg ± 0.11 which contains minimum amount of the polymer that is carbopol 934P and HPMC K4M because they act as bioadhesive polymer due to which the formation of secondary bioadhesion bonds takes place. All the physical parameters of the tablets were practically within control (Table 2).
|
Formulation Codes |
Thickness (mm) ± S.D. |
Hardness (kg) ± S.D. |
Surface pH ± S.D. |
Friability ± S.D. |
Swelling index ± S.D. |
Mucoadhesion time (min) ± S.D. |
Mucoadhesive force (N) ± S.D |
|
F1 |
2.05±0.04 |
5.73 ± 0.11 |
6.96 ± 0.05 |
0.13 ± 0.02 |
2.13 ± 0.05 |
497.01 ± 1.52 |
0.05 ± 0.05 |
|
F2 |
2.06±0.02 |
5.46 ± 0.12 |
6.95 ± 0.05 |
0.25 ± 0.18 |
2.61 ± 0.04 |
512.30 ± 0.57 |
0.08 ± 0.04 |
|
F3 |
2.11±0.07 |
5.86 ± 0.11 |
6.71 ± 0.04 |
0.40 ± 0.14 |
2.99 ± 0.01 |
520.33 ± 0.57 |
0.12 ± 0.47 |
|
F4 |
2.07±0.04 |
7.2 ± 0.11 |
6.90 ± 0.13 |
0.09 ± 0.13 |
1.13 ± 0.05 |
530.61 ± 1.15 |
0.08 ± 0.06 |
|
F5 |
2.02±0.04 |
6.33 ± 0.14 |
6.86 ± 0.05 |
0.12 ± 0.07 |
1.26 ± 0.05 |
526.01 ± 1.16 |
0.11 ± 0.03 |
|
F6 |
2.03±0.03 |
7.27 ± 0.12 |
6.83 ± 0.15 |
0.19 ± 0.11 |
2.40 ± 0.07 |
548.66 ± 1.15 |
0.14 ± 0.01 |
|
F7 |
2.04±0.06 |
7.33 ± 0.11 |
6.81 ± 0.02 |
0.17 ± 0.07 |
0.80 ± 0.06 |
515.33 ± 1.13 |
0.11 ± 0.05 |
|
F8 |
2.04±0.07 |
6.26 ± 0.15 |
6.78 ± 0.02 |
0.07 ± 0.16 |
1.05 ± 0.05 |
522.33 ± 2.51 |
0.14 ± 0.05 |
|
F9 |
2.02±0.05 |
6.82 ± 0.05 |
6.68 ± 0.02 |
0.05 ± 0.02 |
1.31 ± 0.01 |
551.33 ±1.52 |
0.16 ± 0.01 |
Table. 2 Pharmacotechnical parameters of buccoadhesive tablet formulations (F1- F9)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Swelling study
Swelling index was calculated in accordance to time and increased with time as the weight gain by the tablets which are proportionally with the rate of hydration.Swelling study gives an idea of swelling behaviour of the tablet which can be related to the extent of drug release. The swelling of the tablets increased slowly with the increase in the amount of Carbopol which reported in Table 2 and the swelling with the increase in amount of NaCMC. Maximum swelling was obtained in the batch F3 in which the polymer started eroding slowly in the medium. This batch contains NaCMC in combination with HPMC K4M with drug and this batch show this property due to the presence of NaCMC 14.As NaCMC has viscosity less as compared to HPMC K4M and carbopol 934P resulting in low binding forces between the molecules. Since HPMC is a non-ionic polymer, then swelling behaviour of this compound is not affected by the pH15 therefore the swelling rate of HPMC is less than that of carbopol 934P (Table. 2) as the polymer gradually absorbed water due to hydrophilic nature. The increase in swelling index may be attributed to the hydrophilic nature of the polymers owing to which the polymers absorbed water leading to their swelling. Since NaCMC is a hygroscopic material which undergoes humidity condition can absorb a large quantity of water; so the formulation F3 showing the maximum swelling in comparison to other formulation. Thus, an increase in the amount of polymers resulted in an increased swelling index. Swelling phenomenon of the polymers makes strong secondary hydrogen bonding with the buccal mucosa and thus results in mucoadhesion. Moreover, since swelling of polymer is a gradual process, the swelling index increased with the duration of study16, 17.

Fig. 2 Swelling behavior of enalapril maleate buccal tablets
Ex- vivo mucoadhesion time and force
Ex- vivobioadhesive force and time was carried out with the goal of finding the force required to remove the dosage form from the site of application18,19. The bioadhesion time and the bioadhesive force of all the batcheslies between the ranges 497.01 min. ± 1.52 to 551.33min. ±1.52 and 0.05 (N) ± 0.05 to 0.16 (N) ± 0.01 respectively (Table 2). The bioadhesion properties were affected by the type and ratio of the bioadhesive polymers. The different polymers showed the significant differences in their bioadhesion. The highest detachment force was observed with the formulation F9 followed by F8 and F6. The detachment forces of CP934P-NaCMC were greater than those of CP934P- HPMC K4M and NaCMC- HPMC K4M. On the basis of these result further evaluation will be proceeds.
Ex- vivo diffusion study
Ex- vivo diffusion studies were carried out to access the permeation of drug across the buccal mucosa. Diffusion studies were carried out on all the formulations F1 to F9 containing varying amount of polymers. The permeation profile of the drug is shown in the Fig. 2. The percent drug permeated across the porcine buccal mucosa20, 21 at 8 hr. ranged between 66.01± 0.54 % to 91.98 ± 0.58 % (Fig. 3 and Table 3). The formulation F2, F3, F7 and F6 shows the maximum permeation that is 82.96, 86.92, 82.60 and 91.98 % respectively. To study the release kinetics of enalapril maleate from the tablets, the goodness of fit method was applied and different kinetic equations were applied to interpret the release rate from the matrices. In the present study, the linear nature of the curves obtained for zero – order, first order, higuchis model and hixon- crowel model. The different kinetic models that were applied to interpret the drug permeate from the tablets are shown in the Table no. 3. The results of the different formulation showed different models while the formulation F5 and F6 showed the zero order. Hence the best fit with the maximum permeation that is formulation F6 of the enalapril maleate tablet was zero order. According to zero order kinetics drug dissolution from pharmaceutical dosage forms that do not disaggregate and release the drug slowly (assuming that area does not change and no equilibrium conditions are obtained). The overall rate of drug permeates at 8 hr tended to rise with the increasing amount of polymer22.
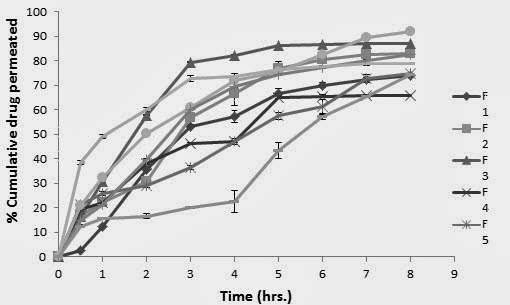
Fig. 3 Ex-vivo diffusion study of the formulations (F1-F9)
Table 3. Percent cumulative drug permeation and release model of the formulations (F1-F9)
|
S. No. |
Time (hr) |
Percent cumulative amount permeated ± S.D. |
|
|
||||||
|
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
||
|
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
2 |
0.5 |
2.5±0.65 |
16.8±0.39 |
16.33±0.01 |
19.33±0.26 |
21.33±0.65 |
20.84±0.38 |
14.91±0.17 |
38.42±0.61 |
12.43±0.33 |
|
3 |
1 |
12.42±0.10 |
22.02±0.24 |
30.59±0.05 |
22.04±0.76 |
25.93±0.75 |
32.53±0.13 |
21.24±0.21 |
49.03±0.73 |
15.48±0.57 |
|
4 |
2 |
35.49±0.07 |
30.69±0.59 |
57.75±0.42 |
38.04±0.18 |
29.27±0.77 |
50.51±0.06 |
39.77±0.31 |
60.14±0.77 |
16.61±0.15 |
|
5 |
3 |
53.31±0.04 |
56.96±2.25 |
79.37±0.21 |
46.27±0.28 |
36.42±1.01 |
60.76±0.36 |
60.14±0.25 |
72.73±0.84 |
20.17±0.96 |
|
6 |
4 |
57.31±0.07 |
66.61±3.06 |
82.34±0.20 | ||||||









