 About Authors:
About Authors:
Hitesh Chopra*
A.S.B.A.S.J.S.M. College Of Pharmacy Bela Ropar.
chopraontheride@gmail.com
Abstract
The first and the second generation type of dosage forms had been found to have lower efficacy of drug reaching to the targeted tissue this lead to need for third generation drug delivery system which contains the liposomes, microspheres, microcapsules, nanodiamonds etc. with these type of drug delivery system it had been found to have better patient compliance and reduced side effects. The present paper deals with the same as there is first formation of Metformin double emulsion and then precipitation of the drug from the polymeric solution. In this study challenge was to encapsulate Metformin with high entrapment efficiency by w/o/o double emulsion solvent diffusion technique using non-aqueous processing media. The primary requirement of this method to obtain microsphere is that selected solvent for polymer must be immiscible with non aqueous processing media, so to fulfill the requirement.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1492
Introduction
The microsphere are the small polymeric structure with size range of 1 to 1000 μm. They may be biodegradeable, bioerodible or non- biodegradeable depending on the polymeric layer that has encapsulated it. The natural polymers includes fats, gums , starches and waxes. The solvent is chosen that is able to dissolve the polymeric layer so that encapsulated drug could be released. The micropshers have smaller size as a result high surface – volume ratio is obtained.
Microspheres manufacture The most important physicochemical characteristics that may be controlled in microsphere manufacture are:
- Partical size and distribution
- Polymer molecular weight
- Ratio of drug to polymer
- Total mass of drug and polymer
[adsense:468x15:2204050025]
MATERIALS :
The Metformin was obtained as gift sample from the J.K. medical agency, Jalandhar. The other chemicals and apparatus was obtained from the college laboratory.
DRUG REVIEW:
4.1 Metformin
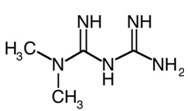
Class: Biguanides
Chemical Name: 1,1-Dimethylbiguanide monohydrochloride
Molecular Formula: C4H11N5.HCl
Brands: Actoplus Met, Avandamet, Fortamet, Glucophage, Glucophage XR, Glucovance, Glumetza, Metaglip, Riomet
Metformin Hydrochloride Solubility:Freely soluble in water; slightly soluble in ethanol (95 per cent); practically insoluble in acetone, in chloroform, in dichloromethane and in ether.
Storage: Store protected from light and moisture.
Description: Metformin is an oral anti diabetic drugin the biguanideclass. It is the first-linedrug of choice for the treatment of type 2 diabetes, in particular, in overweightand obesepeople and those with normal kidney function. Evidence is also mounting for its efficacy in gestational diabetes, although safety concerns still preclude its widespread use in this setting. It is also used in the treatment of polycystic ovary syndrome, and has been investigated for other diseases where insulin resistancemay be an important factor.
Mechanism: Metformin improves hyperglycemia primarily by suppressing glucose production by the liver (hepatic gluconeogenesis). The "average" person with type 2 diabetes has three times the normal rate of gluconeogenesis; Metformin treatment reduces this by over one third. Metformin activates AMP-activated protein kinase (AMPK), an enzyme that plays an important role in insulin signaling, whole body energy balance, and the metabolism of glucose and fats; activation of AMPK is required for Metformin's inhibitory effect on the production of glucose by liver cells. Research further on Metformin's mechanism of action, showed activation of AMPK is required for an increase in the expression of SHP, which in turn inhibits the expression of the hepatic gluconeogenic genes PEPCK and Glc-6-Pase. Metformin is frequently used in research along with AICAR as an AMPK agonist. The mechanism by which biguanides increase the activity of AMPK remains uncertain; however, research suggests that Metformin increases the amount of cytosolic AMP (as opposed to a change in total AMP or total AMP/ATP).
Pharmacokinetics: Metformin has an oral bioavailability of 50–60% under fasting conditions, and is absorbed slowly. Peak plasma concentrations (Cmax) are reached within one to three hours of taking immediate-release Metformin and four to eight hours with extended-release formulations. The plasma protein binding of Metformin is negligible, as reflected by its very high apparent volume of distribution (300–1000 L after a single dose). Steady state is usually reached in one or two days.
Adverse effects
The most common adverse effect of Metformin is gastrointestinal upset, including diarrhea, cramps, nausea, vomiting and increased flatulence; Metformin is more commonly associated with gastrointestinal side effects than most other anti diabetic drugs. The most serious potential side effect of Metformin use is lactic acidosis; this complication is very rare, and the vast majority of these cases seem to be related to comorbid conditions, such as impaired liver or kidney function, rather than to the Metformin itself.
Metformin has also been reported to decrease the blood levels of thyroid-stimulating hormone in people with hypothyroidism, and, in men, testosterone. The clinical significance of these changes is still unknown.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
POLYMER REVIEW:
5.1 Ethylcellulose
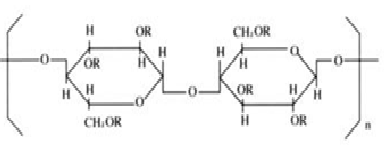
Nonproprietary Names
BP: Ethylcellulose where R = CH3
PhEur: Ethylcellulose
USP-NF: Ethylcellulose
Synonyms: Aquacoat ECD; Aqualon; Ashacel; E462; Ethocel; ethylcellulosum; Surelease.
Empirical Formula and Molecular Weight: Ethylcellulose is partially ethoxylated. Ethylcellulose with complete ethoxyl substitution is C12H23O6 (C12H22O5)n C12H23O5 where n can vary to provide a wide variety of molecular weights. Ethylcellulose, an ethyl ether of cellulose, is a long-chain polymer of b-anhydroglucose units joined together by acetal linkages.
Functional Category Coating agent; flavoring agent; tablet binder; tablet filler; viscosity increasing agent.
|
Use |
Concentration (%) |
|
Microencapsulation |
10.0–20.0 |
|
Sustained-release tablet coating |
3.0–20.0 |
|
Tablet coating |
1.0–3.0 |
|
Tablet granulation |
1.0–3.0 |
Density (bulk):0.4 g/cm3
Moisture content:Ethylcellulose absorbs very little water from humid air or during immersion, and that small amount evaporates readily.
Solubility: Ethylcellulose is practically insoluble in glycerin propylene glycol, and water. Ethylcellulose that contains less than 46.5% of ethoxyl groups is freely soluble in chloroform, methyl acetate, and tetrahydrofuran, and in mixtures of aromatic hydrocarbons with ethanol (95%). Ethylcellulose that contains not less than 46.5% of ethoxyl groups is freely soluble in chloroform, ethanol (95%), ethyl acetate, methanol and toluene.
METHOD OF PREPARATION
Metformin loaded ethylcellulose microspheres were prepared by the w/o/o double emulsion solvent diffusion technique. Required quantity of Metformin and ethylcellulose in ratio 1:1 were dissolved in 5ml a mixture of Acetonitrile and dichloromethane (1:1). The initial w/o emulsion was formed by adding 2 ml of deionized water to the drug polymer solution with constant stirring at 300 rpm for 5 min. the primary emulsion was then slowly added to the light liquid paraffin 50ml containing spam 80 as a surfactant with constant stirring at 500 rpm for 2h. n- hexane 10ml was added to harden the formed microsphere the stirring was then continued for 1h. the liquid paraffin was then decanted of and solution was filtered by vacuum filter. Finally air dried over a period of 12h.
CHARECTERIZATION/ EVALUATION OF MICROSPHERES
Particle size analyzer
Microsphere (50 mg) was suspended in distilled water (5mL) containing 2%w/v of tween 80. To prevent microsphere aggregation, the above suspension is sonicated in water bath and the particle size was expressed as volume mean diameter in micrometer.
Mean particle size =
mean particle size of the fraction * Weight fraction
------------------------------------------------------
Weight fraction
Percentage Yield:
The yield of microsphere was determined by comparing the whole weight of microsphere formed against the combined weight of the polymer and the drug
Percentage yield = mass of microsphere obtained *100
----------------------------------------
Total weight of drug and polymer used
Optical microscopy
This method was used to determine particle size by using optical microscope. The measurement was done under 450x (10x eye piece and 45x objective) and100 particles were calculated.
Swelling index
This technique was used for Characterization of sodium alginate microspheres were performed with swelling index technique Different solution(100mL) were taken such as (distilled water, buffer solution of pH(1.2, 4.5, 7.4) were taken and alginate microspheres (100mg) were placed in a wire basket and kept on the above solution and swelling was allowed at 37 oC and changes in weight variation between initial weight of microspheres and weight due to swelling was measured by taking weight periodically and soaking with filter paper.
RESULTS
|
Formulation |
Ratio of drug and polymer |
Percentage yield (%) |
Theoretical drug content(%) |
Experimental drug content(%) |
Entrapment efficiency(%) |
|
Metformin microsphere |
1:1 |
71.0±1.50 |
50 |
38.0±3.50 |
75.60±0.12 |
DISCUSSION
Preparation of Microsphere
Hydrophilic character of Metformin is likely to preferentially partition out into the aqueous media leading to lower entrapment efficiency when encapsulated using aqueous phase as the processing medium. In this study challenge was to encapsulate Metformin with high entrapment efficiency by w/o/o double emulsion solvent diffusion technique using non-aqueous processing media. The primary requirement of this method to obtain microsphere is that selected solvent for polymer must be immiscible with non aqueous processing media, so to fulfill the requirement Acetonitrile is selected as solvent because it is a unique organic solvent which is polar water miscible and oil miscible. Acetonitrile alone as a solvent did not ensure formation of primary emulsion of aqueous phase in polymer solution when oil as the processing media and on mixing the water miscibility of the Acetonitrile brought about the precipitation of the polymer. Hence a non polar solvent namely dichloromethane was included with Acetonitrile to decrease the polarity of polymer solution.
Particle Size Analysis
The microsphere size increased with increasedpolymer concentration which may be due to significant increase in the viscosity, thus leading to an increase in polymer droplet size and finally a larger microsphere size. The size of microsphere ranges from 254±6.24 to 346±4.21 microns.
Percentage Yield
Percentage yield of microsphere was calculated and the resultsare shown in table.
Conclusion
In conclusion, the attempt to prepare controlled release microspheres of metformin with increased entrapment efficiency was successful, even though the entrapment efficiency was still low compared to the same process reported for other hydrophilic drugs. Further studies are required to find out the exact cause for the difference and to improve the entrapment efficiency.
REFERENCES :
* Bakan J.A.: in The Theory and Practice of Industrial Pharmacy, Lieberman H.A., Lachman L. and Kanig J.L. Eds., p. 412, Lea and Febiger, Philadelphia, PA 1986.
* Rhodes CT. Disperse systems. In: Banker GS, Rhodes CT, editors. Modern pharmaceutics. 2nd ed. New York: Marcel Dekker; 1990. p. 333.
* Garg R , Gupta GD. Progress in Controlled Gastro retentive Delivery Systems. Tropical Journal of Pharmaceutical Research, September 2008; 7 (3): 1055?1066.
* Martindale. The complete drug reference. 32nd ed. Pharmaceutical Press, 1999; pp. 190-191.
* Vyas, S. P., “Targeted and Controlled Drug Delivery Novel Carrier System”, Ist Ed., CBS Publishers and Distributors, New Delhi, 2002 pp. 417-54.
* www.pharmainfo.net/reviews/bioadhesivemicrospheres-review, 24th Nov 2010 Patel JK, Patel RP, Amin AF, Patel MM, 4(6),12/16/2006.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









