About Authors:
Borse V. G.*, Birajdar A. S., Datal A. A.
K. T. Patil College of Pharmacy,
Osmanabad-413501, Maharashtra, India.
*vishalgborse@gmail.com
ABSTRACT :
The objective of this study is to enhance dissolution rate of practically insoluble drug olanzapine, using surface solid dispersion technique. Olanzapine is classified as a thiobenzodiazepine atypical antipsychotic drug used in the treatment of schizophrenia. Surface solid dispersion technique is used to prepare solid dispersion of olanzapine using Crospovidone as carrier. Surface solid dispersion were characterised by saturation solubility studies, drug content and in- vitro dissolution studies. Solubility studies revealed a marked increase in the solubility of olanzapine with an increase in Crospovidone concentration. The best dispersion was selected based on saturation study and release study and evaluated for solid state characterization techniques Differential scanning calorimetry, FT-IR spectroscopy study. The results from DSC and IR indicated that there was no interaction between drug and carriers. Surface solid dispersion of olanzapine shows increase in aqueous solubility 4.42 fold than pure drug. SSD with Crospovidone in 1:9 ratio give highest drug release i.e. 88.87%.Surface solid dispersion is successful technique to improved drug dissolution of olanzapine with the carrier Crospovidone.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1381
INTRODUCTION:
Poor aqueous solubility and bioavailability of drugs into the body after administration are two prime issues which are faced by the pharmaceutical industry at the present time. This problem has been the major problem hampering the release of new chemical entities into the market. Every year more than 50% of the potentially active pharmaceutical ingredients get rejected due to the above stated problems. [1] With the advent of combinatorial chemistry and throughout screening, the number of poorly water soluble compound has dramatically increased. [2] The absorption and bioavailability of such compounds when presented in crystalline state to the GI tract is poor and variable. Poor solubility carry a higher risk of failure during discovery and development, since insufficient solubility may compromise other properties of compound and add undesirable properties, can influence both pharmacokinetic and pharmacodynamic properties of the compound and finally may affect the bioavailability of the compound. Therefore, there is need of a new approach for enhancing solubility of drug. [3],[4]
In the proposed study the, Olanzapine is a relatively new benzodiazepine atypical antipsychotic which belongs to the class of the thiobenzodiazepine and has proven efficacy against the positive and negative symptoms of schizophrenia, bipolar disorder and other psychosis.[5] Olanzapine belongs to BCS class II drug having low solubility (4.27µg/ml) and undergoes extensive first pass metabolismresulting in relatively very low oral bioavailability (60%).[6] In this case, the enhancement of the apparent aqueous solubility of the drug can improve its clinical performance; eventually decrease the dose and side effects. As such the oral absorption of olanzapine is dissolution rate limited thus these are selected as suitable candidates to improve its dissolution characteristics by preparing surface solid dispersions and to study the effect of different carrier at different ratios on the dissolution rate of the poorly water soluble drug olanzapine.
In the present study, Crospovidone used as carrier, which is quickly wicks saliva into the tablet to generate the volume expansion and hydrostatic pressures necessary to provide rapid disintegration in the mouth. Unlike other superdisintegrants, which rely principally on swelling for disintegration, Crospovidone superdisintegrants use a combination of swelling and wicking. When examined under a scanning electron microscope, Crospovidone particles appear granular and highly porous. This unique, porous particle morphology facilitates wicking of liquid into the tablet and particles to generate rapid disintegration. Due to its high crosslink density, Crospovidone swells rapidly in water without gelling. [7]
Surface solid dispersion (SSD) technique has been used to increase the solubility, dissolution and consequently the bioavailability of olanzapine. SSD technique was modified by loading the drug onto the surface of the carrier that can alter the dissolution characteristics of the drug. Deposition of drug on the surface of an inert carrier leads to a reduction in the particle size of the drug, thereby providing a faster dissolution rate. SSD can overcome some of the shortcoming of conventional solid dispersions. The carriers used in surface solid dispersion are water insoluble, porous material and hydrophilic in nature. [8],[9] Surface solid dispersion were prepared by solvent evaporation method with Crospovidoneas carrier in four different drug–carrier ratios and evaluated for different parameters.
[adsense:468x15:2204050025]
MATERIALS AND METHODS:
Materials:
Olanzapine (OLZ) was received as a gift sample from Matrix healthcare Pvt. Ltd., (Ahmadabad, India). Crospovidone, mannitol and aerosil was purchased from Vishal Chemicals, Mumbai. Avicel PH102 was a gift sample from Maple Biotech, Pune.All others chemicals was purchased from Rajesh Chemicals, Solapur. All other reagents used were of analytical grade.UV spectrophotometer (Shimadzu UV1800, Japan), USP dissolution test apparatus (Electrolab TDT 08L, Mumbai, India) and sonicator (Systronics, India) were used.
Methods:
Preparation of physical mixture:
Physical mixtures (PM) were prepared by mixing drug and carrier in geometric proportions using a spatula without applying pressure. The powder was passed through sieve no72#.
Preparation of surface solid dispersions:
The SSDs of olanzapine using carrier Crospovidone was prepared in a different drug to carrier ratio using surface solid dispersion technique. SSDs of olanzapine with Crospovidone containing five different weight ratios (1:1, 1:3, 1:5, 1:7 & 1:9) prepared by solvent evaporation method.
Solvent evaporation method:calculated amount of drug was dissolved into a minimum amount of acetone. Then solution was added to carrier (Crospovidone), while mixing until a homogenous mixture was attained. The obtained slurry was stirred using a magnetic stirrer at room temperature until the solvent (acetone) evaporated completely. The SSDs was transferred to desiccators containing CaCl2 and stored until completely dry. The powder was passed through sieve no72#. [10],[11]. Compositions and code of different SSDs is presented in table I.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Evaluation of surface solid dispersion:
Drug content:
The drug content in each surface solid dispersions and physical mixture was determined by the UV spectroscopic method. An accurately weighed quantity of SSDs and physical mixture, equivalent to 10mg of olanzapine, was dissolved in minimum quantity of methanol and volume was made up to 25ml with methanol. Solutions were kept for 1 hour. The stock solutions were diluted with methanol and analyzed by UV spectrophotometry (Shimadzu 1800) at 226.2nm. [12]
Saturation solubility studies:
Weighed amount of olanzapine (pure drug) and SSDs excess quantity were added to 10ml of distilled water in 30ml into a glass vial. The samples were placed on ultra sonicator for 6hr and kept for 24 hours to achieve equilibrium. An aliquot of each solution was withdrawn and filtered through whatman no.41 filter paper. The content of olanzapine was determined spectrophotometrically at 253.5nm. [13],[14]
Dissolution studies:
The in-vitro dissolution studies for plain olanzapine, physical mixture and SSDs were carried out in USP Apparatus I. Samples equivalent to 10mg of olanzapine filled in empty capsule shell and added to 900mL of 0.01MHCl at 37±0.5°C and stirred at 100rpm. Aliquots of 5mL were withdrawn at specified time intervals. Samples were analyzed spectrophotometrically at 258.3nm.
Similarly, In-vitro dissolution studies of olanzapine, physical mixtures (PM) and SSDs prepared was evaluated in buffer pH 6.8 and distilled water. The samples were analyzed spectrophotometrically at 254.3nm and 253.5nm. Cumulative percent drug dissolved was found out at each interval and graph was plotted between cumulative % drug release v/s time in minutes.
Fourier transforms infrared spectroscopy (FTIR):
The FTIR spectra of samples (olanzapine/carrier/SSDs/physical mixtures)were recorded with FTIR spectrometer (alpha brooker). Samples were prepared by using KBR pellets and scannedin the range of 4500 to 400 cm-1.
Differential scanning calorimetry (DSC):
DSC pattern of samples (olanzapine, carrier and SSDs) was recorded by using Shimadzu TA-60WS thermal analyser. Samples were sealed in aluminium pans, the lids were pierced and DSC thermogram was recorded at heating rate of 200C/min. from 100 to 3000C using nitrogen atmosphere.
Wetting time of prepared tablets:
Circular tissue papers were placed in a petri dish of 6.5cm diameter. 6ml of water containing 0.5% methylene blue, a water-soluble dye, was added to the petri dish. The dye solution was used to identify complete wetting of the tablet surface. A tablet was carefully placed on the surface of the tissue paper in the petri dish. The time required for water to reach the upper surface of the tablets and to completely wet them was noted as the wetting time. These measurements were carried out in triplicates of three. Wetting time was recorded with digital watch. [15]
Dissolution studies of prepared tablets:
The in-vitro dissolution studies for plain olanzapine and SSD tablets were carried out in USP Apparatus II. Tablets were added to 900mL of 0.01M HCl at 37± 0.5°C and stirred at 50rpm. Aliquots of 5mL were withdrawn at specified time intervals. Samples were analyzed spectrophotometrically at 258.3nm. [16]
Dosage form development:
The tablets of selected drug: carrier ratio was prepared by direct compression method. On the basis of dissolution studies surface solid dispersion; OLZ: CPV (1:9) ratio was selected and used for tablet compression. Sufficient quantity of microcrystalline cellulose PH102 (binder), Mannitol (diluent), magnesium stearate (lubricant) and aerosil (glidant) was added and mixed well in a mortar. All the ingredients and SDs were sieved through #60. Each tablet contains 10mg of equivalent weight of olanzapine. The mixture was directly compressed in a 08 station tablet punching machine (CIP, Ahmadabad). Each tablet weighed around 200 mg.
RESULTS AND DISCUSSIONS:
Drug content:
The content of olanzapine in SSDs was assayed by UV spectroscopy. The assay values were found between 96%-99.9% of the theoretical value.
Saturation solubility study:
In the saturation solubility of SSDs, the data indicated increased in solubility of SSDs in distilled water with carrier Crospovidone in the ratio of 1:9 (18.60μg/ml) as compared to pure drug 4.27μg/ml.Increase in the solubility of olanzapine with an increase in concentration of Crospovidone. The solid dispersions have shown the increase in solubility by 4.35 foldas compared to pure drug olanzapine. This might be due to better wetting properties and particle size reduction. Saturation solubility data are shown in figure I.
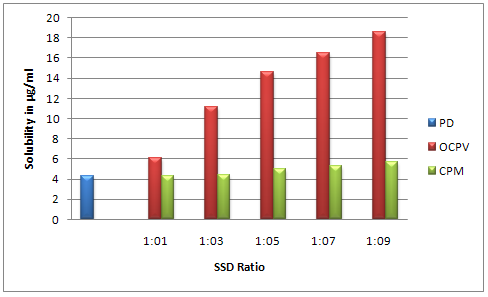
Figure I: Saturation solubility studies
In-vitrodissolution studies:
From the result obtained, it can be seen that dissolution studies in 0.01M HCl, SSD of olanzapine with Crospovidone (1:9) showed maximum percentage drug release 88.87% within one hour, compared to very low dissolution of pure drug i.e. 47.98% and physical mixtures.
Similarly, maximum percentage drug release of SSDs in distilled water was found 72.95% as compared to pure drug show poor dissolution rate i.e. 32.01%. Dissolution studies of SSDs were also performed in buffer pH 6.8. Maximum percent drug release was found 78.72% within one hour, which is found enhanced as compared to pure drug show 37.94% in one hour. Dissolution efficiency of olanzapine in different medium is shown in table II.
Olanzapine surface solid dispersions presented better dissolution performance over corresponding physical mixtures and pure drug. This may be due to an improved wettability of drug particles, a significant reduction in particle size during the formation of surface solid dispersion. [17] The dissolution profiles of olanzapine, physical mixtures and SSDs in 0.01M HCl, buffer pH 6.8 and distilled water are show figure II.SSDs with Crospovidone was characterised by FTIR, and DSC. Hence Surface solid dispersion with Crospovidone in ratio (1:9) was formulated into tablets.
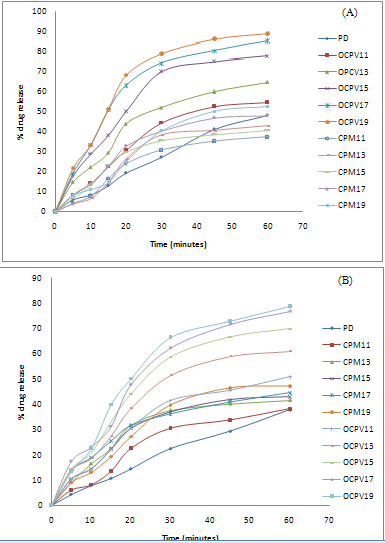
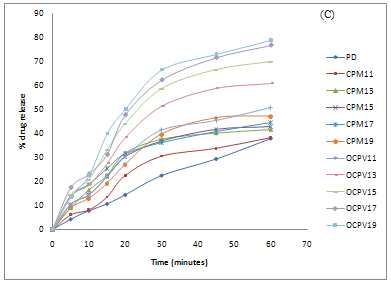
Figure II: Cumulative % drug release from SDs and PM of drug: Crospovidone in (A)0.01M HCl (B) buffer pH 6.8 (C) Distilled water
Fourier transforms infrared spectroscopy:
IR studies were performed on olanzapine, physical mixture and its surface solid dispersion. The spectra of SSD were compared with that of olanzapine. The prominent peaks of olanzapine appears at 1017.27 (C-N), 1582.31 (C=C) and 3228.25 (N-H Stretching). In SSD 1295.93 (C-N), 1490.13 (C=C) and 3214.75 (N-H stretching). The above characteristic peaks also appear in the spectra of physical mixtures of the drug with crospovidone as carrier at the near wave numbers with broadening. From FTIR studies it is very clear that there are no interactions between drug and carrier. All the peaks responsible for the active functional groups were even present in FTIR spectra of drug along with carrier. FTIR spectra are of drug, physical mixture and SSD of olanzapine are shown in figure III.
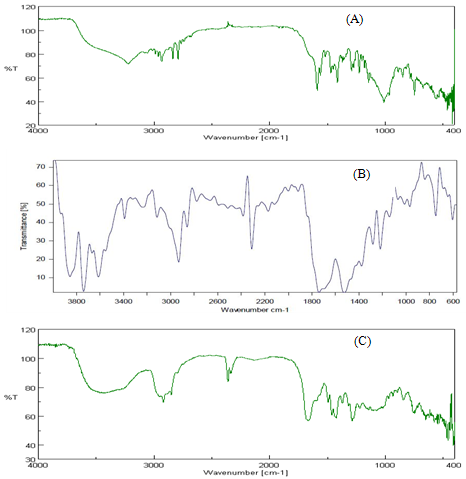
Figure III: IR spectra of (A) olanzapine (B) physical mixture (C) SSD of olanzapine
Differential scanning calorimetry:
The DSC scans of olanzapine, Crospovidone and SSD (OLZ: TN) are shown in figure IV. A sharp single endothermic peak appeared for pure OLZ with following parameter; onset at 193.44oC, peak at 197.52oC. A broad endothermic peak of SSD with crospovidone showed peak at 196.09oC with onset 193.40oC indicates From DSC, it can be concluded that drug and carrier showed no interaction.
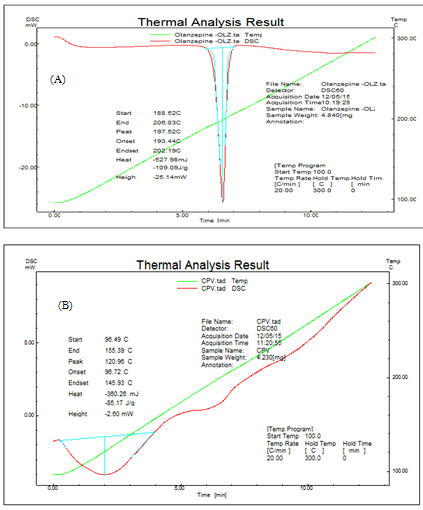
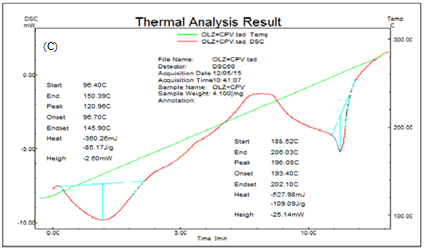
Figure IV: DSC thermogram of (A) olanzapine (B) Crospovidone (C) SSD of olanzapine
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Evaluation of tablets:
Weight variation:
Weight variation data for all the formulations batches indicated no significant difference in the weight of individuals tablets from the average value and weight variation were found within limits.
Hardness:
The result of measured hardness of tablets of the batch is shown in table III; Hardness of all tablets was in range 2.6-4.5kg/cm2.
Thickness:
The result of measured thickness of tablets of the batch is shown in table III; thickness of all tablets was in range 2.9-3.1mm. It was found to within limit as per I.P.
Friability:
The result of measured friability of tablets of the batch is shown in table III; friability of all tablets was within limits i.e. NMT 1%.
In-vitro disintegration time:
The result of measured disintegration time of tablets of the batch is shown in table III; disintegration time of all tablets was in range 13-21sec.
Wetting time:
The result of measured wetting time of tablets of the batch is shown in table III; wetting time of all tablets was in range 18-32sec
Drug content:
The result of drug content of tablets of the batch is shown in table III. Drug content of all tablets was in range i.e. 94-97.1%.
Dissolution studies of prepared tablets:
The results of cumulative % drug release of tablets are shown figure VI. Cumulative % drug release of SSD tablet was compared with pure drug olanzapine tablet. F2 batch (SSD with Crospovidone) tablet showed highest percent drug release i.e. 85.78%, it less as compared with F1 batch (pure drug tablet) i.e. 63.98%.
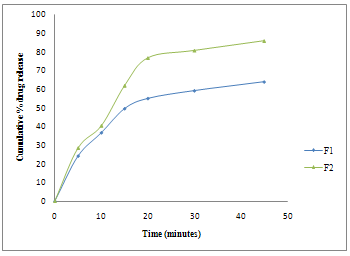
Figure VI: Dissolution studies of prepared tablets
Conclusion:
In present work clearly shows that surface solid dispersion with Crospovidone (1:9) showed marked increase in aqueous solubility & dissolution rate than that of pure drug by using surface solid dispersion technique. Solubility was increased 4.42 fold with Crospovidone carrier. So, Crospovidone was found to be better carrier in enhancing solubility of drug olanzapine. Therefore it can be concluded that improved drug dissolution could be achieved by formulating olanzapine as surface solid dispersions with the carrier Crospovidone.
ACKNOWLEDGEMENT :
I am thankful to my guide Dr. Birajdar A. S. and other staff member for their valuable guidance and suggestions. I am grateful and wish to express my immense sense of thanks and gratitude to ASPM’s K. T. Patil College of Pharmacy, Osmanabad for providing the necessary facilities. I am very grateful toMaple Biotech, Pune for providing me gift sample of pharmaceutical excipient necessary to carry out my research work.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
REFERENCES:
1. Ketan TS. (Drug solubility: importance and enhancement techniques). Faculty of Pharmacy, Institute of Pharmacy Nirma University Ahmadabad, Ahmadabad. 2009.
2. Augsburger L, Hoag S. Pharmaceutical Dosage Forms: Tablets. 3rd ed. New York: Informa Healthcare; 2008. Chapter 2, Approaches for improving Bioavailability of poorly Soluble Drugs; p. 51-104.
3. Karanth H, Shenoy VS, Murthy RR. Industrially feasible alternative approaches in the manufacture of solid dispersions: a technical report. AAPS Pharm Sci Tech. 2006; 7 (4): E1-E8.
4. Kalia A, Poddar M.Solid dispersions: An approach towards enhancing dissolution rate. International Journal of Pharmacy and Pharmaceutical Sciences. 2011; 3(4): 9-19.
5. Sweetman S. Martindale. The complete Drug Reference. 34th ed, Pharmaceutical Press; 2005. p. 909-911.
6. Amidon GL, Kasim NA, Whitehouse M, Ramachandran C, Bermejo M, Stavchansky S, et al. Molecular properties of who essential drugs and provisional biopharmaceutical classification. Molecular pharmaceutics. 2003; 1(1): 85-96.
7. Mohanachandran PS, Sindhumol PG, Kiran T.S. Superdisintegrants: an overview. International Journal of Pharmaceutical Sciences Review and Research. 2011; 6(1):105-109.
8. Dixit RP, Nagarsenkar MS. In vitro and in vivo advantage of celecoxib surface solid dispersion and dosage form development. Ind J Pharm Sci. 2007; 69(3): 370-377.
9. Patel B, Parikh RH, Swarnkar D. Enhancement of dissolution of telmisartan through use of solid dispersion technique-surface solid dispersion. Journal of Pharmacy and Bioallied Sciences. 2012; 4: 64-68.
10. Rao M, Mandage Y, Thanki K, Bhise S. Dissolution improvement of simvastatin by surface solid dispersion technology. Dissolution Technologies. 2010; May: 27-34.
11. Derle DV, Pawar AY, Patel JS, Rathi MN, Kothawade PI. Solubility enhancement of aceclofenac by solvent deposition method. Int J Pharm Tech Res. 2010; 2(1): 843-846.
12. Modi A, Tayade P. Enhancement of dissolution profile by solid dispersion (kneading) technique. AAPS Pharm Sci Tech. 2006; 7 (3): 1-6.
13. Das A, Kumar AN, Mohanty B, Panda S.Solubility and dissolution enhancement of etoricoxib by solid dispersion technique using sugar carriers. International Scholarly Research Network Pharmaceutics. 2011; 1-8.
14. Jani R, Jani K, Setty MC, Patel D. Preparation and evaluation of solid dispersions of aceclofenac. International Journal of Pharmaceutical Sciences and Drug Research. 2009; 1(1): 32-35.
15. Sahoo S, Mishra B, Biswal P, Panda O, Jana JK. Fast dissolving tablet: as a potential drug delivery system. Drug Invention Today. 2010; 2(2):130-133.
16. Indian Pharmacopoeia 2007, Volume 3. The Indian Pharmacopoeia Commission. Ghaziabad. 857.
17. Elbary AA, Salem HF, Maher HE. In vitro and in vivo evaluation of glibenclamide using surface solid dispersion approach. British Journal of Pharmacology and Toxicology. 2011; 2(1): 51-62.
ABBREAVIATIONS (figures and tables legends):
OLZ: Olanzapine
SSD: Surface Solid Dispersion
PM: Physical Mixture
PD: Pure drug
CPM: Olanzapine with Crospovidone Physical Mixture
OCPV: SSD of Olanzapine with Crospovidone
F1: Pure drug tablet
F2: SSD tablet
Tables:
Table I: Composition and code of different surface solid dispersions
|
Sr. No. |
SSD Codes |
Methods |
Composition |
Ratio (w/w) |
|
1 |
OCPV11 |
Solvent Evaporation |
OLZ:CPV |
1:1 |
|
2 |
OCPV13 |
Solvent Evaporation |
OLZ:CPV |
1:3 |
|
3 |
OCPV15 |
Solvent Evaporation |
OLZ:CPV |
1:5 |
|
4 |
OCPV17 |
Solvent Evaporation |
OLZ:CPV |
1:7 |
|
6 |
OCPV19 |
Solvent Evaporation |
OLZ:CPV |
1:9 |
|
7 |
CPM11 |
Physical mixture |
OLZ:CPV |
1:1 |
|
8 |
CPM13 |
Physical mixture |
OLZ:CPV |
1:3 |
|
9 |
CPM15 |
Physical mixture |
OLZ:CPV |
1:5 |
|
10 |
CPM17 |
Physical mixture |
OLZ:CPV |
1:7 |
|
11 |
CPM19 |
Physical mixture |
OLZ:CPV |
1:9 |
Table II: Dissolution efficiency of olanzapine in different medium
|
Codes |
Dissolution medium |
||||||
|
Distilled water |
Buffer pH 6.8 |
0.01M HCl |
|||||
|
DE30 |
DE60 |
DE30 |
DE60 |
DE30 |
DE60 |
||
|
Olanzapine |
20.52 |
32.01 |
22.53 |
37.94 |
27.10 |
47.98 |
|
|
OCPV19 |
65.53 |
72.95 |
66.39 |
78.72 |
79.20 |
88.87 |
|
OCPV19- Olanzapine with Crospovidone in 1:9 ratio
Table III. Evaluation tests for prepared tablets
|
Tablet Codes |
Thickness (Avg.) (mm) |
Hardness (Avg.) (mm) |
Friability (%) (Avg.) |
Disintegration Time (Sec.) |
Wetting Time (Sec.) |
Drug Content (%) |
Q45 |
|
F1 |
3.1± 0.4 |
4.5±0.5 |
0.686±0.4 |
21 |
32 |
98.3 |
63.98 |
|
F2 |
2.9± 0.3 |
2.6±0.4 |
0.947±0.6 |
13 |
18 |
93.1 |
85.98 |
F1– Pure drug tablets,
*mean ±SD (n=3)
F2- SSD tablets
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









