 About Authors:
About Authors:
Virag A. Shah1*, Tarashankar Basuri1, Vishal S. Modi1, Rohit R. Shah2, Dhananjay S. Ghodke2
1 SSR College of pharmacy, Silvassa, U.T of Dadra & Nagar Haveli-396230
2 Appasaheb Birnale College of Pharmacy, Sangli, Dist: Sangli, Maharashtra- 416416
*viragshah687@gmail.com
Abstract:
Cyclodextrins (CDs) due to their inclusion formation ability finds wider applicability in Novel Drug Delivery Systems as well as in Pharma industries. This review highlights and focuses on history of CD molecules, their chemistry, need for derivatizations of CDs, production method, CDs inclusion complexes and various methods used for complexation. The article also focuses on various effects of CDs on drug solubility and dissolution, bioavailability, stability, and safety. Some important drug delivery systems which utilize CDs are also summarized with a brief overview on recent advances in CDs.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1727
Introduction
Carbohydrates are probably the most abundant organic substances in nature and from very ancient time they have been used for shelter, clothing and food. Carbohydrates are processed through fermentation process from thousands of years and observed for their enzymatic degradation. It is now known to mankind that the processing of carbohydrates leads to formation of mixtures of monosaccharides, disaccharides and various oligosaccharides, such as linear and branched dextrins, and under certain conditions small amount of cyclic dextrins or cyclodextrins (CDs) are also being reported during these degradation processes. In 19th century technological advances laid the foundation of carbohydrate chemistry and by the middle of the century a number of relatively pure carbohydrates such as glucose, fructose, mannose, lactose, sucrose, cellulose, cotton, starch etc. were known to chemists in Europe1. A short chronological summary on the development of cyclodextrins is given in Table 1.
Table 1: Chronological summary on the development of Cyclodextrins
|
Years |
Events |
|
1891 |
Villiers publishes his discovery of cellulosine (cyclodextrin). |
|
1903 |
Schardinger publishes his first paper on α- and β-dextrins. |
|
1924 |
Methylation of cyclodextrins first described, later both Freudenberg and Meyer Delius (1938) and Szejtli (1980) prepared different grades of methylated cyclodextrins. |
|
1928–1932 |
The ability of cyclodextrins to form complexes with various organic compounds discovered. |
|
1935 |
Freudenberg and Jacobi discovered γ-cyclodextrin. |
|
1938–1952 |
The chemical structure of α-, β- and γ cyclodextrin elucidated by Freudenberg, Cramer, Borchert, French and Rundle. |
|
1948–1951 |
Formation and structure of cyclodextrin (CD) inclusion complexes discovered. |
|
1953 |
The first cyclodextrin patent entitled “Method for preparation of inclusion compounds of physiologically active organic compounds” was issued in Germany to Freudenberg, Cramer and Plieninger. |
|
1954 |
Cramer’s book on inclusion complexes published. |
|
1957–1965 |
French describes the existence of large natural cyclodextrins with up to 12 glucose units |
|
1965 |
Higuchi and Connors publish their article on classification of complexes based on their phase-solubility profiles. |
|
1976 |
The parent α- and β-cyclodextrin officially approved as food additives in Japan. |
|
1976 |
The world’s first pharmaceutical product, prostaglandin E2/β-cyclodextrin (Prostarmon ETM sublingual tablets), marketed in Japan by Ono Pharmaceutical Co. |
|
1981 |
The first International Symposium on Cyclodextrins was organized and held in Budapest by Szejtli. |
|
1983–1985 |
Brauns and Muller (Europe) and Pitha (USA) filed patents on 2-Hydroxypropyl β-cyclodextrin. |
|
1988 |
Piroxicam/β-cyclodextrin tablets (Brexin®) marketed by Chiesi Pharmaceutical (Italy). |
|
1990 |
Stella and Rajewski filed patent on sulfobutyl ether β-cyclodextrin. |
Emerging of cyclodextrins:
The emerging of cyclodextrins was explained by Professor Jozsef Szejtli and was classified on the basis of chemical and industrial development of cyclodextrins into three stages.
1. Discovery period
2. Exploratory period
3. Utilization period
These three developmental phases closely follow the technological advances that took place during the last century, first in analytical instrumentation and then in biotechnology.
1. Discovery period:
A. Villiers a French scientist in 1991 firstly reported the formation of some unidentified substance at fermentation of starch. His experimental results indicated that the substance was a dextrin1. He determined its composition to be (C6H10O5)2·3H2O and named it “cellulosine”. After the discovery of dextrin 15 years later, an Austrian microbiologist, Franz Schardinger, found that there are microorganism responsible named Bacillus macerans which is responsible for the conversion of starch molecules into two distinct crystalline substances. The properties of the reported substances were found to be similar to already reported dextrin’s so he name them as α-, and β-dextrin. In those years cyclodextrin (CD) were named Schardinger dextrin’s in his honor2.
2. Exploratory period:
After the discovery period in 1938, Freudenberg and his team started exploration the structure of these two dextrins and found it to be cyclic in nature. In 1954 Cramer describes all the basic structural and physicochemical characteristics of α-, β- and γ-cyclodextrin, including their chemical structure, cavity size, solubility, reactivity, complexing abilities, and their effect on the chemical stability of guest molecules. In 1980 it was discovered that certain type of amylase, cyclodextrin glucosyl transferase (CGTase), could detach a turn of the polysaccharide helix and link the two ends of the fragment to give a cyclic dextrin. Many microorganism produce glucosyl transferases but only few produce CGTase. Schardinger had shown that Bacillus macerans formed cyclodextrin, i.e. that it produces CGTase. During the exploratory period various strains of bacteria were screened with regard to their ability to form cyclodextrins and since then several microorganisms have been shown to produce CGTase, including strains of Bacilli, strains of the genus Micrococcus and strains of the genus Klebsiella.Exploratory period ends with the development of various method of preparation of cyclodextrins for lab scale production1.
3. Utilization period:
The utilization of cyclodextrins started soon after studying the toxicology which proved that any toxicity attributing to CDs may be due to:
- Complexed impurities
- Inadequate form of administration
- Extreme dosing.
In 1981 the first International Symposium on Cyclodextrins was organized. Derivatizations of CD molecules started leading to the production of more than 100 other CD derivatives 2.
[adsense:468x15:2204050025]
Chemistry of Cyclodextrins:
CDs are ‘bucket-like’ or ‘cone-like’ rigid molecules, with a central core, the size of which differs in different cyclodextrin molecules. The arrangement of hydroxyl groups within the molecule gives the external surface hydrophilic characteristic, while internal surface of the core is hydrophobic. This type of chemistry of cyclodextrin allows the guest molecule to fit in the core and form an inclusion complex4.
Chemically they are cyclic oligosaccharides containing six (α-CD), seven (β-CD), eight (γ-CD), nine (δ-CD), ten (ε-CD) or more D-(+) glucopyranose units attached by α-(1, 4) glucosidic bonds5. Chemical structure of three main types of cyclodextrins α, β and γ are shown in fig. 1.

Fig.1 Chemical structures of α-, β- and γ-cyclodextrin
The natural cyclodextrins, in particular β-cyclodextrin, are of limited aqueous solubility meaning that complexes resulting from interaction of lipophiles with these cyclodextrins can be of limited solubility resulting in precipitation of solid cyclodextrin complexes from water and other aqueous systems. In fact, the aqueous solubility of the natural cyclodextrins is much lower than that of comparable acyclic saccharides. This may be due to relatively strong intermolecular hydrogen bonding in the crystal state. Substitution of any of the hydrogen bond-forming hydroxyl groups, even by lipophilic methoxy functions, results in dramatic improvement in their aqueous solubility6.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Need for Derivatizations of Cyclodextrins:
Every glucopyranose unit in cyclodextrin molecule has three free hydroxyl groups which differ both in their functions and reactivity. The relative reactivities of C2 and C3 secondary, and the C6 primary hydroxyls depend on the reaction conditions (like pH, temperature, reagents etc). In β-CD, 21 hydroxyl groups can be modified substituting the hydrogen atom or the hydroxyl group by a large variety of substituting groups like alkyl-, hydroxyalkyl-, carboxyalkyl-, amino-, thio-, tosyl-, glucosyl-, maltosyl-, etc.
The aim of such derivatizations may be:
* To improve the solubility of the CD derivative (and its complexes);
* To improve the fitting and/or the association between the CD and its guest, with concomitant stabilization of the guest, reducing its reactivity and mobility;
* To attach specific (catalytic) groups to the binding site (e.g., in enzyme modeling); or
* To form insoluble, immobilized CD-containing structures, polymers (e.g., for chromatographic purposes).
The following is the list of important CDs derivative which find wider industrial applications:
· Randomly methylated β-CD (RM- β-CD)
· 2-hydroxypropyl β-CD (HP- β-CD)
· Chlorotriazinyl β-CD
· Glucosyl and maltosyl β-CD
· Sulfobutylether β-CD (SBE- β-CD)2
Physicochemical properties of cyclodextrins7:
Various physicochemical properties of cyclodextrin are summarized in Table 2.
Table 2: Physical and chemical properties of natural cyclodextrin α, β and γ
|
Characteristics |
α |
β |
γ |
|
No. of glucose unit |
6 |
7 |
8 |
|
Internal diameter (nm) |
0.47-0.53 |
0.60-0.65 |
0.75-0.83 |
|
Depth of cavity (nm) |
0.79 |
0.79 |
0.79 |
|
Molecular weight |
972 |
1135 |
1297 |
|
Solubility in water (gm/100ml) |
14.5 |
1.85 |
23.2 |
|
Cavity diameter in A° |
4.7-5.3 |
6-6.5 |
7.5-8.5 |
|
Volume of cavity (approx) in A° |
174 |
262 |
472 |
|
Crystal forms |
Hexagonal plates |
Monoclonic parallelogram |
Quadratic prism |
|
Crystal water (% w/w) |
10.2 |
13.2-14.5 |
8.13-17.7 |
|
Number of water molecules taken by cavity |
6 |
11 |
17 |
|
Hydrophobic interaction |
CD-cavity |
CD-cavity |
CD-cavity |
|
Hydrogen bond |
Glucose-OH |
Glucose-OH |
Glucose-OH |
Production of cyclodextrin:
The process of production of cyclodextrins can be explained in two phases. First phase consist of production of the enzyme CGTasethat converts starch to cyclodextrins, which may be obtained from Bacillus macerans, Klebsiella pneumonia and Alcalophyl Bacillus. This enzyme is contained in crude form in the cell-free filtrate of the culture media. The filtrate has to be concentrated and the enzyme partially purified. Partially prehydrolyzed starch is then treated with CGTaseto obtain a mixture of α, β, and γ-cyclodextrins together with a series of linear dextrins. Second phase involves the separation of cyclic and linear products of enzymatic degradation of starch for which different procedures have been reported. Separation of the mixture may be achieved by addition of appropriate organic solvents. E.g. tetrachloroethane, a process resulting in the precipitation of the crystalline complexes are decomposed by steam distillation which removes the solvent. The mixture of cyclodextrin is then fractionated by repeated dissolution and reprecipitation with organic compounds that form crystalline complexes only with one of the cyclodextrins. Alternative processes for isolation of cyclodextrins from the conversion mixture use treatment of the mixture with another enzyme, known as glucoamylase. This enzyme rapidly converts linear dextrin’s molecules to glucose and the remaining cyclodextrin may then be isolated in a simple fashion. Thus, β-cyclodextrin, due to its low solubility, can be crystallized directly from these solutions. While such a process avoids the use of organic solvents, it does have disadvantage: only a fraction of the β-cyclodextrin crystallizes while a considerable part together with the well soluble α- and γ-cyclodextrins, remains in the mother liquor. Under technical conditions a higher yield & lower production costs can be obtained by controlling the enzyme conversion. Thus the enzymatic conversion is made in the presence of toluene, then by using an appropriate pH, temperature & reaction time, the main product is β-cyclodextrin. This enrichment is due to the formation of insoluble complex of β-cyclodextrin with toluene, a process that continuously removes the β-cyclodextrin from the system & thus shifts the equilibrium towards the product. Contamination of the final product with toluene is not serious; β-cyclodextrin with less than 3 ppm of aromatics can be made in this way, a contamination that is lower than 10 ppm allowed by regulations of health agencies. Similarly, the addition of decane or decanol leads to an increased production of α-cyclodextrin. The decanol concentration in the crystalline α-cyclodextrin is below 5 ppm, a concentration that is admissible in products used in human consumption1, 8.
Cyclodextrin inclusion complexation:
Cyclodextrin inclusion complexation phenomenon is like a “host-guest interaction”. In this interaction cyclodextrin molecule act as host and the drug molecule to be entrapped in host cavity act as guest. Cyclodextrin complexation involves entrapment of one molecule of guest in cyclodextrin cavity in contrast to other encapsulation methods, which involve entrapment of more than one guest. For formation of complex with cyclodextrin, variety of non-covalent forces like Vander wall forces, hydrophobic interaction, and dipole movement are responsible. In majority of cases only a single guest molecules is entrapped in the cavity. For high molecular weight molecules, more than one molecule of cyclodextrins can bind to the guest 6, 9, 10. The central CD cavity provides a lipophilic microenvironment into which suitably sized drug molecules may enter and include. During drug/CD (D/CD) complex formation no covalent bonds are formed or broken in aqueous solutions and the complexes are readily dissociated. The rates for formation and dissociation of drug/CD complexes are very close to the diffusion controlled limits and drug/CD complexes are continuously being formed and broken apart11. The value of K1:1 is most lies between 50 and 2000M-1 with a mean value of 130, 490 and 350 M-1 for α-CD, β-CD and γ-CD, respectively12, 13, 14. Non-cyclic oligosaccharides and polysaccharides are also known to form water-soluble complexes with lipophilic water-insoluble compounds. Like non-cyclic oligosaccharides, it is also possible that CDs form non-inclusion complexes where, for example, the hydroxyl groups on the outer surface of the CD molecule form hydrogen bonds with the drug of interest. It has been reported that α-CD forms both inclusion and non-inclusion complexes with dicarboxylic acids and that the two types of complexes coexist in aqueous solutions. However, the inclusion-type of guest/host CD complexes is probably much more common than the non-inclusion CD complexes15.For the preparation of complex, many solvents are used, but generally water is preferred as a solvent for complexation. The cavity of cyclodextrin in non-polar and it favours non-polar area of guest molecule. Water gives driving force for formation of complexation. Not all guests are sufficiently soluble in water. It is not necessary that complete solubilization of guest should be done. Small amount of guest must be soluble to form a complex. Sometimes water miscible solvents in small quantities are helpful for dissolution of guest, which enhances complexation reaction. After addition of the dissolved guest to the solution of cyclodextrin, either guest may be dissolved or suspended in the form of high precipitate. Excess quantity of solvent if added, results in decrease in driving force for complexation reaction by reducing the difference in polarity between the bulk solution and cyclodextrin cavity which ultimately leads to little or no complexation but good solubilization of guest. Heat can destabilize the inclusion complex. Complexation stability depends on the temperature of guest and it must be optimized for every guest 6, 9, 10.
Methods for preparation of cyclodextrin inclusion complexation:
Several methods were reported for formation of cyclodextrin inclusion complexes. The methods generally preferred are as follows:
1. Kneading:
Kneading process involves the formation of paste of cyclodextrin with drug molecule by using small quantity of either water or ethanol to form kneaded mass which is dried at 45°C and pulverized16.
2. Melting:
Excess quantity of the drug is melted and mixed with powdered or fine cyclodextrin. After cooling, excess quantity of drug is removed by washing with weak complex forming solvent. The method restricted to sublimable guest molecules like menthol17.
3. Co-evaporation:
Aqueous solution of cyclodextrin is added to the alcoholic solution of drug molecules, which is stirred for sometimes, evaporated at room temp until dried mass is obtained, pulverized, sieved and finally fraction is collected18.
4. Microwave irradiation:
This method uses microwave for rapid organic synthesis and reactions, which require shorter reaction time and higher product yield19.
5. Freeze drying:
The required stoichiometric quantity of CD and drug were weighed and added to aqueous solution of cyclodextrin and this suspension is stirred magnetically for 24 hours. The resulting mixture is freeze dried at –60°C for 24 hours20.
6. Spray drying:
In this method, CD solution is prepared generally in ethanol: water 50% v/v. To this drug is added and resulting mixture is stirred for 24 hrs at room temperature and solution is spray dried by optimizing the conditions-air flow rate, flow rate of solution, atomizing air pressure, inlet temperature, outlet temperature etc. Product obtained is passed through micrometer granulometric sieve to obtained powder product21.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Effect of CDs on important drug properties in formulations:
1. Effect on Drug Solubility and Dissolution:
The apparent drug solubility and dissolution of poorly water-soluble drugs can be improved by forming cyclodextrin inclusion complexation or solid dispersion. Various application of CD as solubilizing agent is summarized in Table 3. It act as hydrophilic carriers for drugs with inadequate molecular characteristics for complexation, or as tablet dissolution enhancers for drugs with high dose, with which use of a D/CD complex is difficult 22.
Table 3: Effect of cyclodextrin on solubility and dissolution rates of poorly soluble drugs
|
Drug |
CD Used |
Solubility |
Dissolution |
References |
|
Ganciclovir |
HP-β-CD |
↑ |
- |
23 |
|
Rifampicin |
γ-CD |
↑ |
- |
24 |
|
Carbamazepine |
β-CD |
↑ |
↑ |
25 |
|
Nimesulide |
γ-CD, α-CD, β-CD |
↑ |
↑ |
26 |
↑ = Increase
- = No effect
2. Effect on Drug Bioavailability:
Cyclodextrin inclusion complexation enhances the bioavailability of insoluble drugs by increasing its drug solubility, dissolution and drug permeability. This is achieved by making the drug available at the surface of the biological barrier, e.g., skin, mucosa, or the eye cornea, from where it partitions into the membrane without disrupting the lipid layers of the barrier5. Mode of penetration enhancement by CD is shown in Fig. 2.List of drug showing improvement in bioavailability is shown in Table 4.
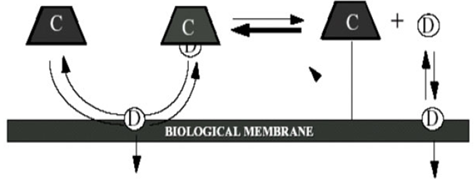
Fig. 2 Mode of penetration enhancement by CD5
Table 4: List of drug showing improvement in bioavailability
|
Drug |
CD Used |
References |
|
Flutamide |
β-CD, HP-β CD |
27,28 |
|
Ketoprofen |
β-CD |
29 |
|
Baicalein |
HP-β-CD |
30 |
|
Acyclovir |
β-CD |
31 |
3. Effect on Drug Stability:
Cyclodextrins protect several labile drugs against dehydration, hydrolysis, oxidation and photodecomposition which can improve the stability and thus increase the shelf life of drugs. Table 5Summarize the effects of cyclodextrin on drug stability. The enhancement in drug stability by cyclodextrin is the result of inhibition of drug interaction with vehicles and inhibition of drug bioconversion at the absorption site. By providing a molecular shield, cyclodextrin complexation encapsulates labile drug molecules at the molecular level and thus insulates them against various degradation22.
Table 5: Effect of CD on drug stability22
|
Drug |
CD used |
Effect |
|
Promethazine |
HP-β-CD |
Photostability |
|
Rutin |
HP-β-CD |
Stability against hydrolysis |
|
Doxorubicin |
HP-β-CD |
Stability to acid hydrolysis and photodecomposition |
4. Effect on Drug Safety:
The increased drug efficacy and potency, caused by CD-increased drug solubility, indirectly reduces the drug toxicity by making the drug effective at lower doses (Table 6.).
Table 6: Increase in drug safety by cyclodextrin Complexation
|
Drug-CD complex |
Effect |
References |
|
Ganciclovir/β-CD |
Increase in drug potency and reduction in toxicity |
32 |
|
Phenytoin/ 2-HP-β-CD |
Reduced tissue irritation |
33 |
|
Piroxicam/β-CD |
Reduction in severity of gastrointestinal side effects |
34 |
Applications of cyclodextrin in various drug delivery systems5:
The following is a list of drug delivery systems were various cyclodextrins finds application.
1. Oral Drug Delivery
2. Parenteral Drug Delivery
3. Ocular Drug Delivery
4. Nasal Drug Delivery
5. Sublingual Drug Delivery
6. Rectal Drug Delivery
7. Pulmonary Drug Delivery
8. Controlled Drug Delivery
9. Colon-Specific Drug Delivery
10. Peptide & Protein Delivery
11. Gene & Oligonucleotide Delivery
12. Dermal & Transdermal Delivery
13. Brain Drug Delivery
Recent advances in cyclodextrin application:
Some of the recent advances associated with cyclodextrin application are summarized as follows:
Cyclodextrin Polymer-Cationic Derivative Complex as a Carrier for DNA Delivery System:
For DNA delivery system the carriers used are generally term as vectors.The vectors used for these delivery are mainly of two types:
1. Viral vectors
2. Non-viral vectors
Viral vectors are mostly widely studied vectors for this type of drug delivery.Remaining are the non-viral vectors. The most widely studied non-viral vectors consist of use of structually well defined & majority of them are cationic such as polymer, lipids & peptides. These molecules are studied due to similarity in function which is essential for transfection. One such molecule is cationic adamantyl derivatives connectors which form an inclusion complex with cyclodextrin polymer (polyβCD) & this result in the formation of “Polycation”. The DNA part is then is attach via coopertive electrostatic interaction to the polycation. The negative charged phosphate group of DNA & cation charge of the connectors forms the ternary complex called as “Polyplex”. This system provides some advantages which are:
1. Cyclodextrin lowers the cell toxicity & increases transfection
2. It can be used for cell targetting
The various adamantyl cationic derivatives were used for the complexation with cyclodextrin polymer (polyβCD). The stability of the system strongly dependent upon the chemical stucture of adamantyl connector, nature, the space between adamantyl moiety & the charge valency. The physicochemical properties of three different adamantyl connector have been studied by different technique
(i) The affinity between Ada connectors and polyβCD using fluorimetry,
(ii) The DNA complexation and compaction using complementary experiments of gel electrophoresis
(iii)The structure of the polyplexes by SANS.
Further improvements of this vector should involve cell targeting. This can be easily realized by adding another adamantane derivative bearing a specific ligand recognized by cell receptors35.
Grafting of Magnetic Nanoparticles with Cyclodextrin for Delivery of Hydrophobic Drug:
Nanoparicles are widely studied for targetted drug delivery. A novel magnetic nanocarriers, with cyclodextrin–citrate & gum arabic modified magnetic nanoparticles (GAMNPs) for the delivery of hydrophobic drug was fabricated by grafting the citarte-modified cyclodextrin onto the GAMNPs. Gum arabic is a natural polymer with a mucoadhesive property that has some desirable features of a controlled drug delivery system. On the other hand, cyclodextrins form a whole new family of pharmaceutical excipients that have a doughnut-shaped structure with a hydrophilic outer surface and a lipophilic cavity, where poorly water-soluble molecules can be incorporated. Therefore, grafting cyclodextrin molecule on the GAMNPs may lead to a drug carriers that possess following properties:
1. Allows a controlled release of a bioactive substance
2. Can form non-covalent inclusion complexes with a wide variety of lipophilic drug molecules allowing the solubilization, stabilization, and transport of hydrophobic drugs
3. Can be magnetically guided to the local site at the specified time for dosage and elimination.
In this study Ketoprofen a NSAIDS, a poorly soluble drug is incorporated with cyclodextrin-citrate GAMNPs.The use of CD−citrate−GAMNPs as the carrier might be a promising strategy for the dosing of hydrophobic drugs like ketoprofen. The newly fabricated nanocarrier injected into the patient via the circulatory system could serve a major purpose through drug targeting to inflammation sites to maintain appropriate concentrations while at the same time reducing cost, overall dosage, and unwanted side effect
The results of ketoprofen inclusion and release experiments indicate that this system seems to be a very promising vehicle for the administration of hydrophobic drugs36.
Self-Assembling Hydrogel based on β-CD/Cholesterol Inclusion Complex:
Hydrogel are hydrophilic polymer networks, which absorb substantial amount of water. Hydrogel can be prepared by either one of the two methods:
1. Chemical cross-linking
2. Physical cross-linking
Chemical cross-linking methods involve cross-linking between the polymer chains. In this cross-linking the agent used may damage the loaded drug. So, physical cross-linking is preferred over chemical cross-linking.
In physical cross-linking the network formed is retained by non-permanent, reversible interaction between polymer chains. Also physical interaction provides a self-assembling property which is an attractive system for drug delivery.
In this research work a novel self-assembling hydrogel system has been formed based on star-shaped 8-arm PEG modified with either β-CD or Cholesterol inclusion complex with the aid of physical cross-linking. The resulting hydrogel formed are thermoreversible & gel characteristics can be adjusted by changing the solid content & other parameters. Star-shaped polymers exhibit smaller hydrodynamic radii and a lower viscosity compared with linear polymers which is beneficial for future pharmaceutical applications, such as injectable in-situ gelling devices. PEG is a biocompatible, non-immunogenic polymer, and is widely used in the products which have been approved by the US Food and Drug Administration. Consequently, this hydrogel system based on the formation of β-cyclodextrin/cholesterol inclusion complexes is a very attractive candidate for further pharmaceutical and biomedical applications37.
Future Prospects:
CDs, as a result of their inclusion forming ability and other versatile characteristics, are continuing to have different applications in different areas of drug delivery and pharmaceutical industry. Since CDs continue to find several novel applications in drug delivery, we may expect these polymers to solve many problems associated with the delivery of different novel drugs through different delivery.
ACKNOWLEDGEMENTS:
The author wishes to thank Prof. D.D. Chougule-Principal, Prof R.R. Shah-H.O.D-Pharmaceutics, and Mr. D.S. Ghodke-Lecturer, Appasaheb Birnale College of Pharmacy-Sangli for their valuable guidance and support, Shri. B.B. Chougule-Librarian for providing library facility and last but not the least we wish to thank to all our friends and colleagues for their valuable support.
REFERENCES
1. Loftsson T, Duchene D. Cyclodextrins and their pharmaceutical applications, International Journal of Pharmaceutics 2007, 329, 1–11.
2. Szejtli J. Past, present, and future of cyclodextrin research, Pure Applied Chemistry 2004, 76(10), 1825-1845.
3. Szejtli J. Cyclodextrins and their inclusion complexes, Akademiai Kiad, Budapest 1982, 120-122.
4. Wade A, Weller PJ (2nd ed.), Handbook of Pharmaceutical Excipients, The Pharmaceutical Press, London. 1994; 362-366.
5. Challa R, Ahuja A, Ali J, Khar RK. Cyclodextrin in drug delivery: An updated Review, AAPS PharmSciTech 2005, 6(2), 329-357.
6. Szejtli J. Cyclodextrin Technology, Kluwer Academic, Dordrecht, 1988.
7. Zukowski J, Sybilska D, Bojarski J. Application of α- and β-cyclodextrin and heptakis (2, 6-di-O-methyl)-β-cyclodextrin as mobile phase components for the separation of some chiral barbiturates into enantiomers by reversed-phase high-performance liquid chromatography, Journal of chromatography 1986, 364, 225-232.
8. Kobayashi S, Kainuma K, Suzuki S. A new preparation method of cyclodextrin, Journal of Japanese Society Starch of Sciences 1975, 22, 6-10.
9. Sham S, Bhaskar C, Prjakta S, Cyclodextrin application in different route of administration, Acta Pharma 2005, 55, 139-156.
10. Baboota S., Khanna R., Agarwal S. Cyclodextrin in drug delivery systems: An update, pharmainfo.net/, 2003, (05).
11. Stella V, Rao V, Zannou E, Zia V. Mechanisms of drug release from cyclodextrin complexes, Advanced Drug Delivery, 1999, 36, 3-16.
12. Stella V, Rajewski R. Cyclodextrins: their future in drug formulation and delivery, Pharmaceutical Research 1997, 14, 556-567.
13. Connors K. Population characteristics of cyclodextrin complex stabilities in aqueous solution, Journal of Pharmaceutical Sciences 1995, 84, 843-848.
14. Rao V, Stella V. When can cyclodextrins be considered for solubilizing purposes, Journal of Pharmaceutical Sciences 2003, 92, 927-932.
15. Gabelica V, Galic N, De Pauw E, On the specificity of cyclodextrin complexes detected by electrospray mass spectrometry, Journal of the American Chemical Society 2002, 13, 946-953.
16. Ammar H, Salma H, Ghorab M. Formulation and biological evaluation of glimepiride-cyclodextrin-polymer systems, International Journal of Pharmaceutics 2006, 309, 129-138.
17. Marie W, Maggie A. Solid state studies of drug-cyclodextrin inclusion complexes in PEG-6000 prepared by new method, European Journal of Pharmaceutical Sciences 1999, 8, 269-281.
18. Cirri M, Maestrelli F, Corti G. Simultaneous effect of cyclodextrin complexation, pH, and hydrophilic polymers on naproxen solubilization, Journal of Pharmaceutical and Biomedical Analysis 2006, 42, 126-131.
19. Xianu H, Fei T, Zhijun J. Preparation and study of the 1:2 inclusion complex of carvedilol with β-cyclodextrin, Journal of Pharmaceutical and Biomedical Analysis 2004, 34, 517-523.
20. Moyano J, Gines J, Arias M, Rabasco A. Study of dissolution characteristics of oxazepam via complexation with β-cyclodextrin; International Journal of Pharmaceutics 1995, 114, 95-102.
21. Castillo J, Canales J, Garcia J, Lastres J, Bolas









