Novartis Cosentyx shows improvement in hidradenitis suppurativa in trials
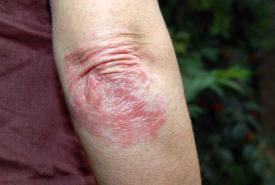
Novartis announced the results from two pivotal, Phase III studies, in which Cosentyx (secukinumab) demonstrated rapid and sustained relief from the common clinical signs and symptoms of moderate-to-severe hidradenitis suppurativa (HS) with a favorable safety profile.
The data were presented as a late-breaking abstract at the 31st European Academy of Dermatology and Venereology (EADV) Congress.