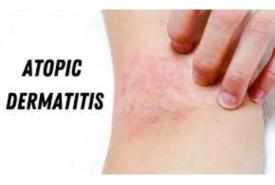
Show cause notices issued to 143 pharma firms after inspections
The Central Drugs Standard Control Organisation along with state licensing authorities has conducted risk-based inspections of 162 pharmaceutical firms and issued show-cause notices in 143 cases, Union Health minister Mansukh Mandaviya said on Tuesday.

















.png)


