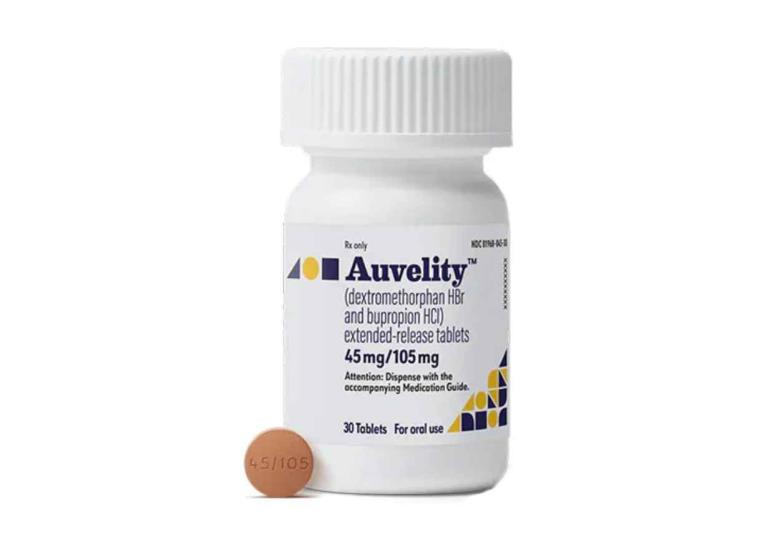
Axsome Therapeutics announces FDA Approval of AUVELITY
Axsome Therapeutics, Inc a biopharmaceutical company developing and delivering novel therapies for the management of central nervous system (CNS) disorders, today announced that the U.S. Food and Drug Administration (FDA) has approved AUVELITYTM (dextromethorphan HBr -bupropion HCl) extended-release tablets for the treatment of major depressive disorder (MDD) in adults.

















.png)


