Amneal Receives USFDA approval for Albuterol Sulfate Inhalation Aerosol
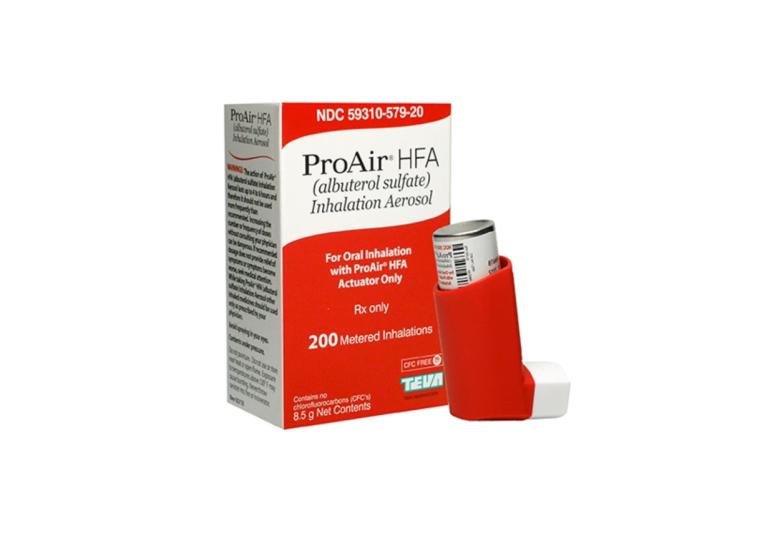
Amneal Pharmaceuticals, Inc. announced that the U.S. Food and Drug Administration has approved the Companys albuterol sulfate inhalation aerosol (90 mcg per actuation). The product is the generic equivalent of PROAIR® HFA (albuterol sulfate inhalation aerosol), a registered trademark of Teva Respiratory LLC.