 (1st June, 2014); Dr Harsh Vardhan, Union Minister for Health and Family Welfare, has asked for thorough streamlining of systems in the distribution of free generic drugs to government health institutions at all levels throughout the country. The Free Drug Programme’s formal launch would be preceded by working out all logistical details with respect to procurement and distribution in consultation with the state governments.
(1st June, 2014); Dr Harsh Vardhan, Union Minister for Health and Family Welfare, has asked for thorough streamlining of systems in the distribution of free generic drugs to government health institutions at all levels throughout the country. The Free Drug Programme’s formal launch would be preceded by working out all logistical details with respect to procurement and distribution in consultation with the state governments.
 (Business Wire India; 5th June, 2014); A new report by the Advanced Medical Technology Association (AdvaMed) demonstrates the lack of awareness among consumers about medical devices.
(Business Wire India; 5th June, 2014); A new report by the Advanced Medical Technology Association (AdvaMed) demonstrates the lack of awareness among consumers about medical devices.
The findings of a survey of 1300 Indians from across 17 states conducted by AdvaMed – an association of medical device manufacturers leading the effort to provide medical technology to the world – reveals that although an overwhelming 72% of respondents use medical devices, 89% say that they do not know enough about them.


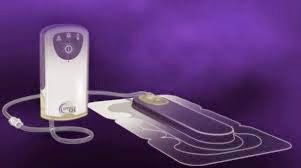 (Business Wire India; 5th June, 2014); Kinetic Concepts, Inc. announced today that a new study, published in the International Wound Journal, showed the Prevena™ Incision Management System used over closed incisions for the first six to seven days reported a significantly lower infection rate than conventional dressings in patients undergoing cardiac surgery via median sternotomy. Post-sternotomy wound infection is a serious, potentially fatal complication of cardiac surgery which often requires additional surgery, and is associated with significantly increased health care costs.
(Business Wire India; 5th June, 2014); Kinetic Concepts, Inc. announced today that a new study, published in the International Wound Journal, showed the Prevena™ Incision Management System used over closed incisions for the first six to seven days reported a significantly lower infection rate than conventional dressings in patients undergoing cardiac surgery via median sternotomy. Post-sternotomy wound infection is a serious, potentially fatal complication of cardiac surgery which often requires additional surgery, and is associated with significantly increased health care costs. (Business Wire India; 3rd June, 2014); Bristol-Myers Squibb and Syngene International, India’s largest contract research organization, today announced a five-year extension of their drug discovery and development collaboration in India. Financial terms were not disclosed.
(Business Wire India; 3rd June, 2014); Bristol-Myers Squibb and Syngene International, India’s largest contract research organization, today announced a five-year extension of their drug discovery and development collaboration in India. Financial terms were not disclosed. (15th May, 2014); Researchers from Center of Advanced European Studies and the University Department of Growth and Reproduction find out large amount of endocrine-disrupting chemicals interfere with human sperm function and that may have a negative impact on fertilization. The study indicates that the chemicals might evoke changes in swimming behaviour at the wrong time and wrong place, hinder navigation of sperm towards the egg and hamper penetration into the protective egg coat.
(15th May, 2014); Researchers from Center of Advanced European Studies and the University Department of Growth and Reproduction find out large amount of endocrine-disrupting chemicals interfere with human sperm function and that may have a negative impact on fertilization. The study indicates that the chemicals might evoke changes in swimming behaviour at the wrong time and wrong place, hinder navigation of sperm towards the egg and hamper penetration into the protective egg coat. (15th May, 2014); Researchers have shown that neurons generated from the skin cells of people with schizophrenia behave unfamiliar in early developmental stages. They used new stem cell research technology for same and they believe that this research may provide new ideas to detect and potentially cure the disease early.
(15th May, 2014); Researchers have shown that neurons generated from the skin cells of people with schizophrenia behave unfamiliar in early developmental stages. They used new stem cell research technology for same and they believe that this research may provide new ideas to detect and potentially cure the disease early. (14th May, 2014); Today, the Wellcome Trust has signed a concordat on openness on animal research in the UK. There are 72 other organisations across the scientific sector, including universities, charities, commercial companies, research councils, umbrella bodies and learned societies are joining in concordat.
(14th May, 2014); Today, the Wellcome Trust has signed a concordat on openness on animal research in the UK. There are 72 other organisations across the scientific sector, including universities, charities, commercial companies, research councils, umbrella bodies and learned societies are joining in concordat.





