(30th June, 2014); Union Health Minister Dr. Harsh Vardhan held a four hour long meeting with top experts at the world famous Centre for Disease Control and Prevention (CDC) at Atlanta on 28th June.
(Business Wire India; 9th July, 2014); HealthCare Global Enterprises Ltd, The Specialist in Cancer Care, organized a press conference on the topic cancer patients suffer due to non availability of drugs.


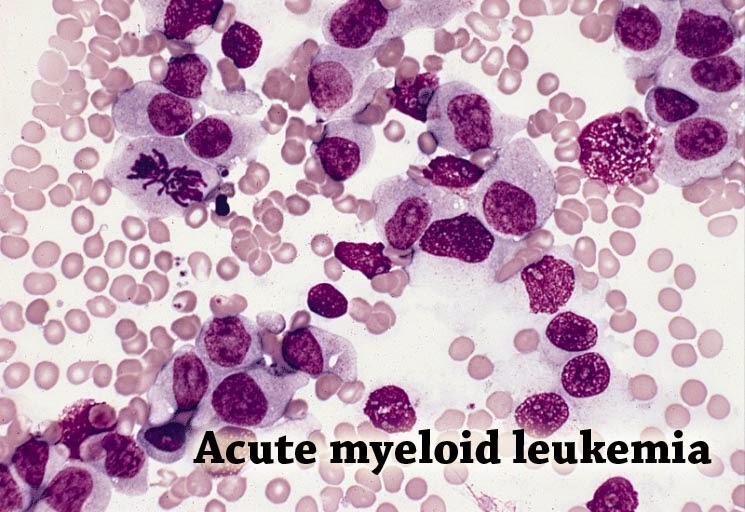 (Business Wire India; 16th June, 2014); Acute myeloid leukaemia (AML) is a devastating form of blood cancer that mainly affects people over 60 years old. For older patients who receive chemotherapy, the median survival is less than a year. Even though AML is considered rare, it accounts for around 18,860 new cases and 10,000 deaths in the US each year and is the most frequent cause of leukaemia-related deaths.
(Business Wire India; 16th June, 2014); Acute myeloid leukaemia (AML) is a devastating form of blood cancer that mainly affects people over 60 years old. For older patients who receive chemotherapy, the median survival is less than a year. Even though AML is considered rare, it accounts for around 18,860 new cases and 10,000 deaths in the US each year and is the most frequent cause of leukaemia-related deaths.





