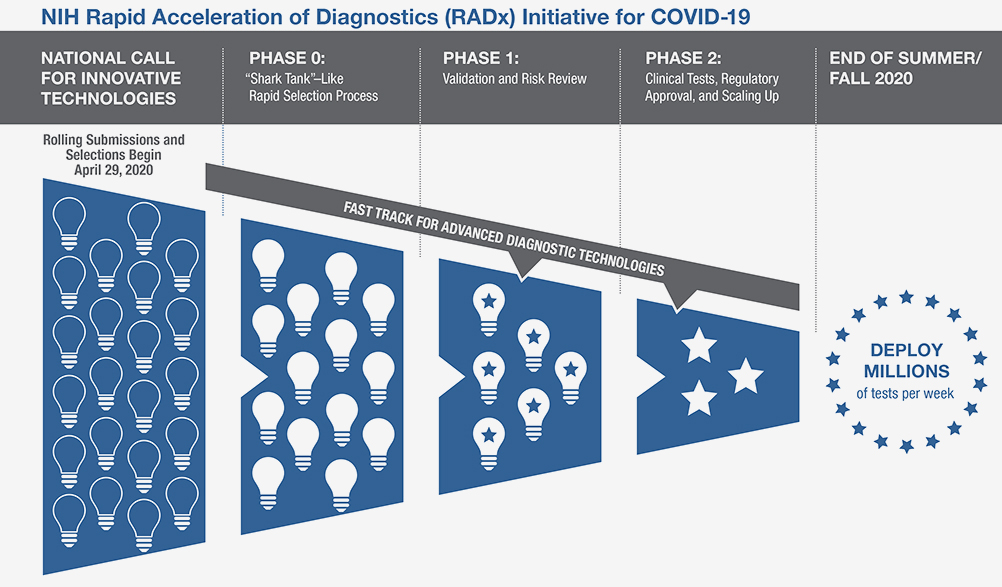
The National Institutes of Health announced a new initiative aimed at speeding innovation, development and commercialization of COVID-19 testing technologies, a pivotal component needed to return to normal during this unprecedented global pandemic. With a USD 1.5 billion investment from federal stimulus funding, the newly launched Rapid Acceleration of Diagnostics (RADx) initiative will infuse funding into early innovative technologies to speed development of rapid and widely accessible COVID-19 testing.
At the same time, NIH will seek opportunities to move more advanced diagnostic technologies swiftly through the development pipeline toward commercialization and broad availability. NIH will work closely with the U.S. Food and Drug Administration, the Centers for Disease Control and Prevention and the Biomedical Advanced Research and Development Authority (BARDA) to advance these goals.
The stimulus investment supercharges NIH’s strong research efforts already underway focused on prevention and treatment of COVID-19, including the recently announced planned Accelerating COVID-19 Therapeutic Interventions and Vaccines public-private partnership to coordinate the international research response to the pandemic.
As part of this initiative, NIH is urging all scientists and inventors with a rapid testing technology to compete in a national COVID-19 testing challenge for a share of up to USD 500 million over all phases of development. The technologies will be put through a highly competitive, rapid three-phase selection process to identify the best candidates for at-home or point-of-care tests for COVID-19.
Finalists will be matched with technical, business and manufacturing experts to increase the odds of success. If certain selected technologies are already relatively far along in development, they can be put on a separate track and be immediately advanced to the appropriate step in the commercialization process. The goal is to make millions of accurate and easy-to-use tests per week available to all Americans by the end of summer 2020, and even more in time for the flu season.
<< Back to Pharma News
Subscribe to PharmaTutor News Alerts by Email