Eminence Business Media organized the Pharma GMP, GCP & Quality Management Webinar on May 14th-15th, 2020.
Get the latest news from world and India’s leading pharmaceutical companies Pharma Industry, pharmaceutical marketing, generic drugs, and Complete news for Pharmacy and Life Sciences professionals.

Eminence Business Media organized the Pharma GMP, GCP & Quality Management Webinar on May 14th-15th, 2020.

Patients with a vascular condition called abdominal aortic aneurysm did not benefit from taking the common antibiotic doxycycline for two years to shrink the aneurysm when compared to those who took a placebo, according to a Vanderbilt University Medical Center (VUMC) study published in the Journal of the American Medical Association (JAMA).

Loss of smell (anosmia) and changes in taste (dysgeusia) were strongly associated with SARS-CoV-2, according to a Canadian study published in CMAJ (Canadian Medical Association Journal).

A potential blood-based biomarker for Alzheimer's and other neurodegenerative diseases seems even more promising thanks to new research from a Massachusetts General Hospital-led study. According to this team's work, neurofilament light chain (NfL) has great potential as a biomarker for early detection of Alzheimer's disease and could be also useful for monitoring treatment response for that condition.
Giving beneficial bacteria to stressed mothers during the equivalent of the third trimester of pregnancy prevents an autism-like disorder in their offspring, according to a new animal study by University of Colorado Boulder researchers.

A new study shows that people with a rare genetic disease that causes bleeding in the brain have gut microbiomes distinct from those without the disease. Moreover, it is the molecules produced by this bacterial imbalance that cause lesions to form in the brains of these patients.
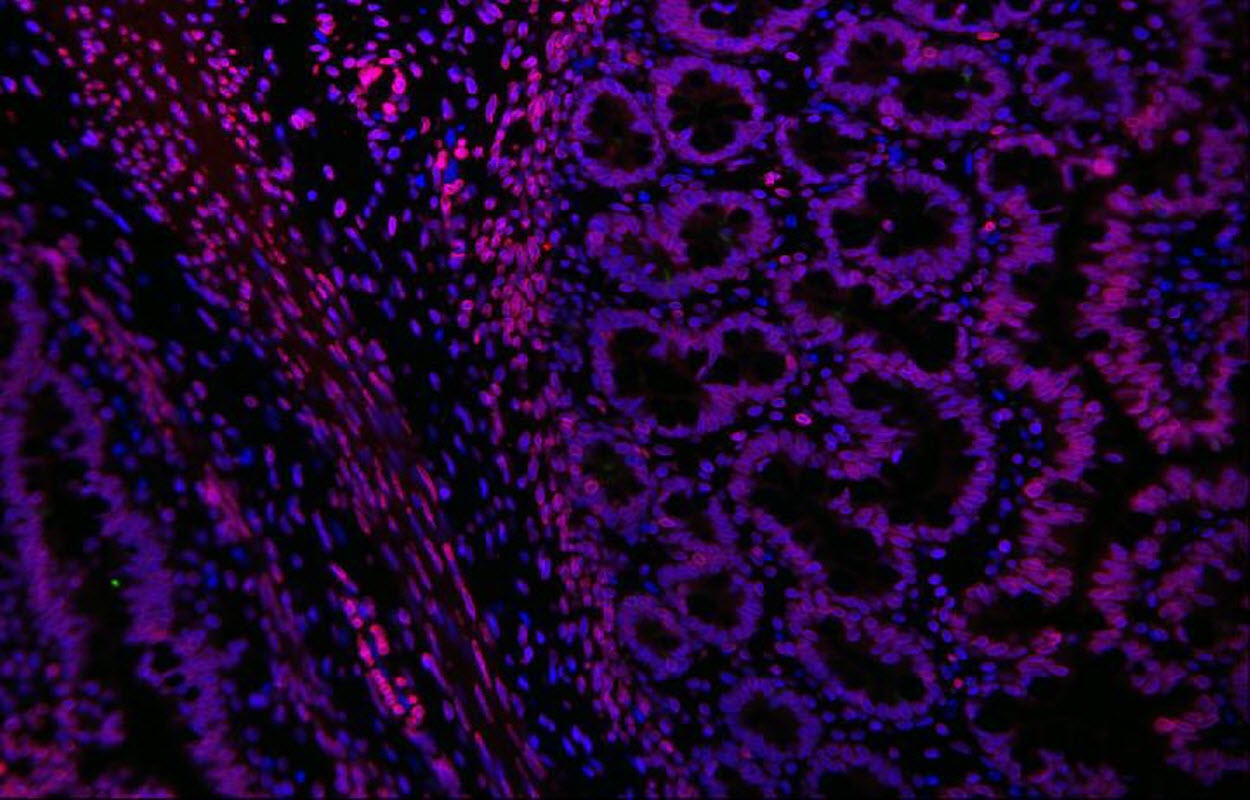
Caption : Human intestine, engineered in the laboratory of Tracy Grikscheit, MD.
Babies with Hirschsprung's disease are born with an incomplete or absent gut nervous system. Children's Hospital Los Angeles investigator Tracy Grikscheit, MD, runs a laboratory that investigates the therapeutic potential of tissue engineering - the induced growth of healthy tissue using stem cells. In a new study, Dr. Grikscheit successfully grew a fully functional gut nervous system - or ENS - in a pre-clinical model. While not yet available clinically, the finding brings surgeons like Dr. Grikscheit one step closer to helping babies in need.
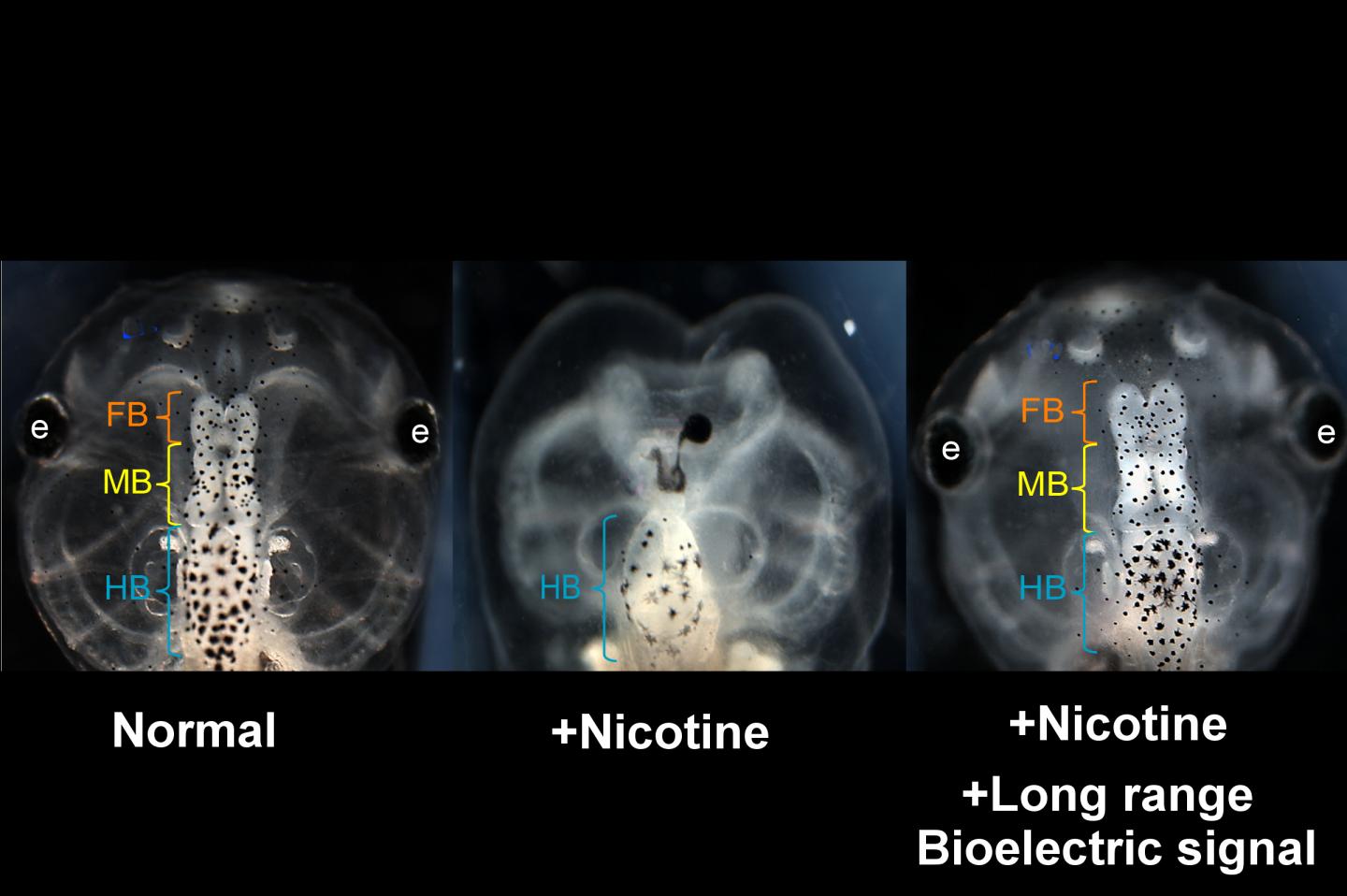
Caption : Nicotine induced defects in the frog embryo brain (center) can be rescued by transplanting an HCN2 expressing patch on the embryo far from the brain. Treated embryos are observed to have normal brain morphology and function (right). View of normal embryo head is shown at left. Similar results are seen when nicotine-exposed embryos are treated with ionoceutical drugs. (FB = forebrain; MB = midbrain; HB = hindbrain)
Researchers led by biologists at Tufts University have discovered that the brains of developing frog embryos damaged by nicotine exposure can be repaired by treatment with certain drugs called "ionoceuticals" that drive the recovery of bioelectric patterns in the embryo, followed by repair of normal anatomy, gene expression and brain function in the growing tadpole. The research, published today in Frontiers in Neuroscience, introduces intervention strategies based on restoring the bioelectric "blueprint" for embryonic development, which the researchers suggest could provide a roadmap for the exploration of therapeutic drugs to help repair birth defects.

Two novel biomarkers have been found to correlate with improved outcomes with immunotherapy in metastatic breast cancer and may help to identify the patients most likely to benefit from this treatment, according to exploratory studies reported at the ESMO Breast Cancer Virtual Meeting 2020. The biomarkers are an increase in the number of programmed death ligand-1 (PDL1/CD274) genes measured by copy number alteration (CNA) and the PD-L1 combined positive score (CPS), which assesses PD-L1 expression on both tumour and immune cells.