Pharma News
Get the latest news from world and India’s leading pharmaceutical companies Pharma Industry, pharmaceutical marketing, generic drugs, and Complete news for Pharmacy and Life Sciences professionals.
-
-
-
E-Market Place- the online Market place for Health & AYUSH products launched Virtually by Dr. S. Eswara Reddy, Joint Drugs Controller, CDSCO and Mr Anil Khaitan, Former President, PHD Chamber.
-
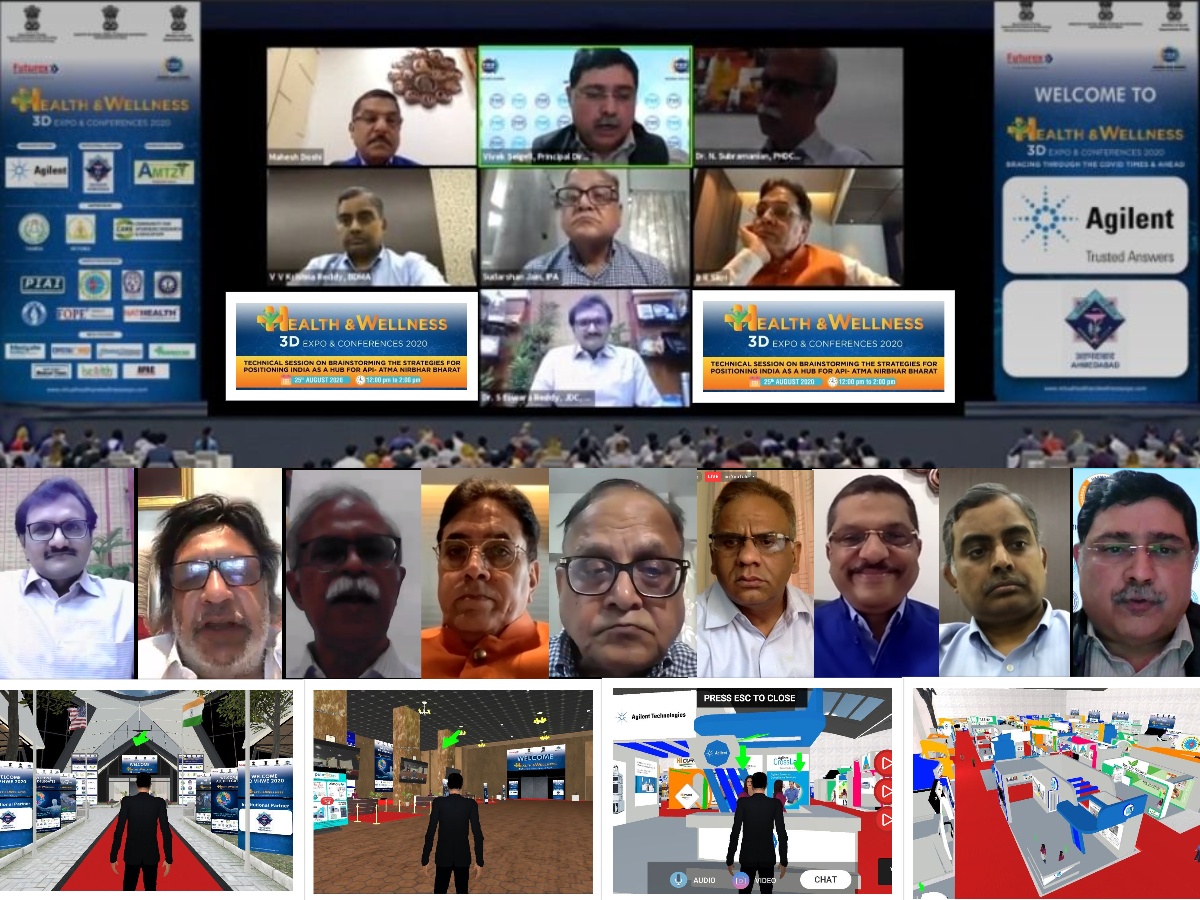
India should be the Hub for API and the API schemes are to boost the indigenous manufacturing of API said by Dr. S Eswara Reddy, Joint Drugs Controller, CDSCO.
-

The use of antibiotics in people with COVID-19 could result in increased resistance to the drugs' benefits among the wider population, a new study suggests.
-
-

Momenta Pharmaceuticals Inc (Momenta) announced that it has entered into a definitive agreement with Johnson & Johnson (J&J). According to the agreement, J&J will acquire Momenta for USD 52.50 per share in an all-cash transaction.
-
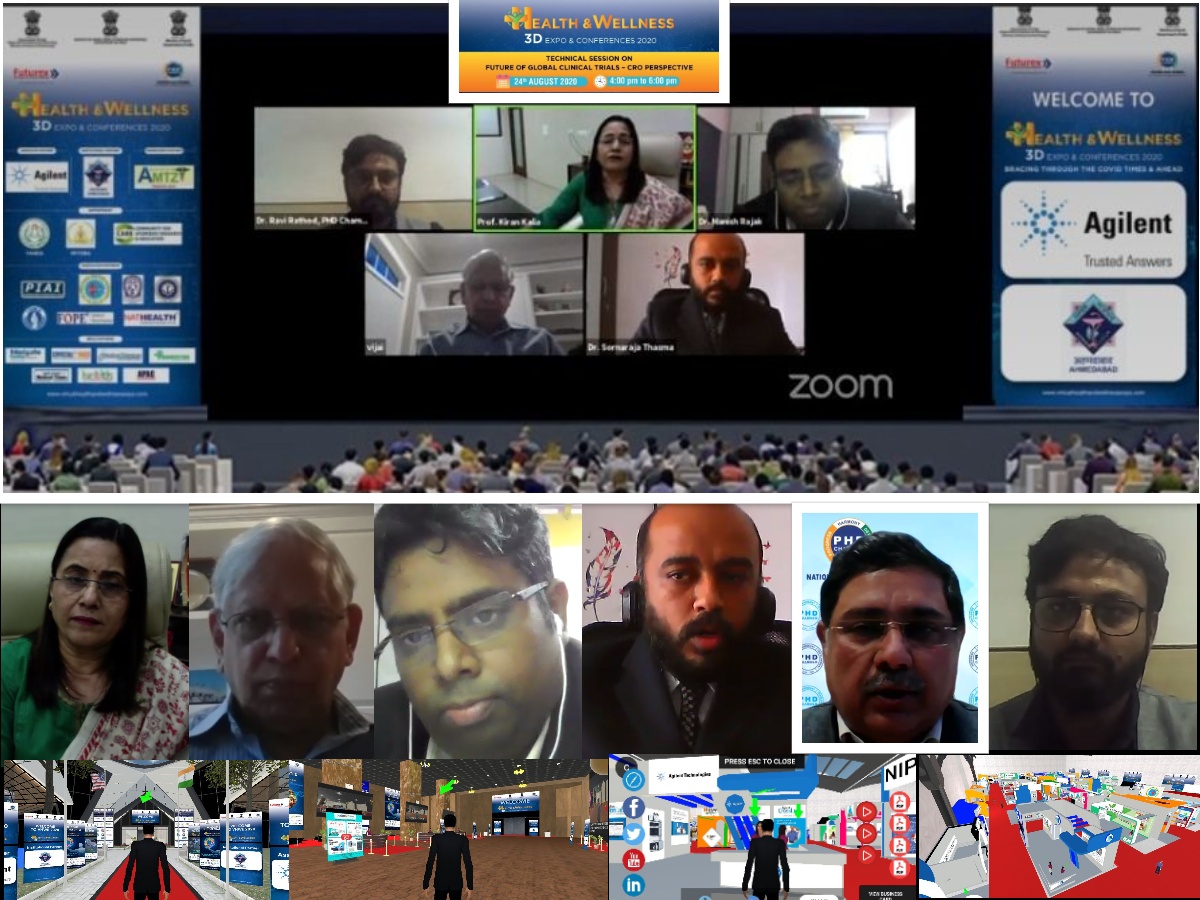
Industry needs to have a transparency to gain the patient trust and awareness needs to be created through all channels and platforms said by Prof. Kiran Kalia, Director, NIPER, Ahmedabad.
-

US Food & Drug Administration (US FDA) has approved the Abbreviated New Drug Application (ANDA) filed by Granules Pharmaceuticals, Inc (GPI)., a wholly owned foreign subsidiary of Granules India Limited for Ramelteon Tablets, 8 mg.
-
WHO working with CCRH, Ministry of AYUSH, GoI for working on the education standards and benchmark in Homeopathy colleges and CCRH and there is a need to allow adjuvant treatment of Homeopathy with Modern Medicine for Covid 19.