Get the latest news from world and India’s leading pharmaceutical companies Pharma Industry, pharmaceutical marketing, generic drugs, and Complete news for Pharmacy and Life Sciences professionals.

CDSCO (Central Drugs Standard Control Organization) has developed draft guidelines for vaccine development in light of the present pandemic situation in the country. Development of vaccine is a complex activity which involves multidisciplinary research and generation of adequate laboratory, nonclinical and clinical data to ensure their safety, efficacy and quality. India is one of the major producers of vaccines in the world which cater the major portion of requirements of many vaccines globally.
The fight against Typhoid fever is all set to get a major fillip with a team of scientists led by Dr. Amit Kumar from Indian Institute of Technology (IIT)-Indore, finding new ways to tackle Salmonella enterica, the pathogenic bacterium that causes the fever.
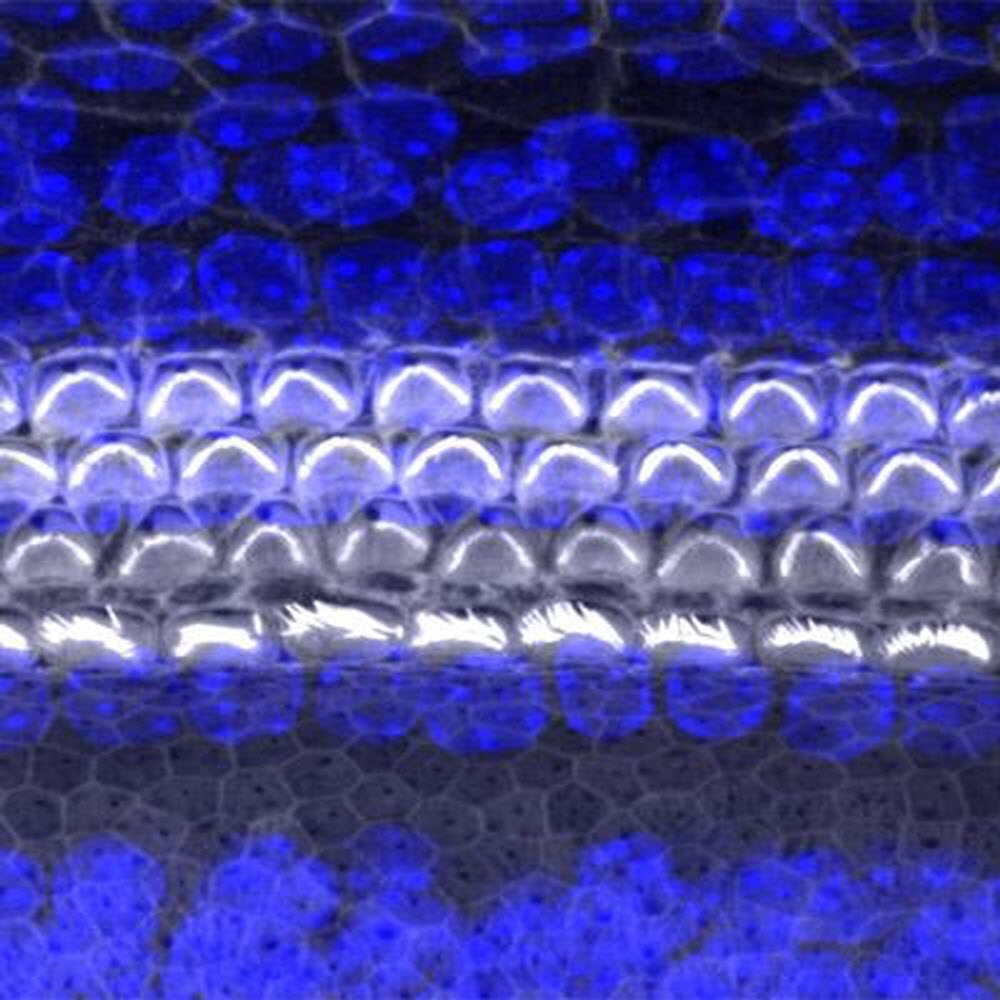
Caption : A surface view of the organ of hearing (cochlea) from a mouse, using confocal microscopy. The sensory cells are named hair cells because of their apical projections (stereocilia) which move from stimulation by sound. Credit : University of Maryland School of Medicine