Scientists make breakthrough in understanding how penicillin works
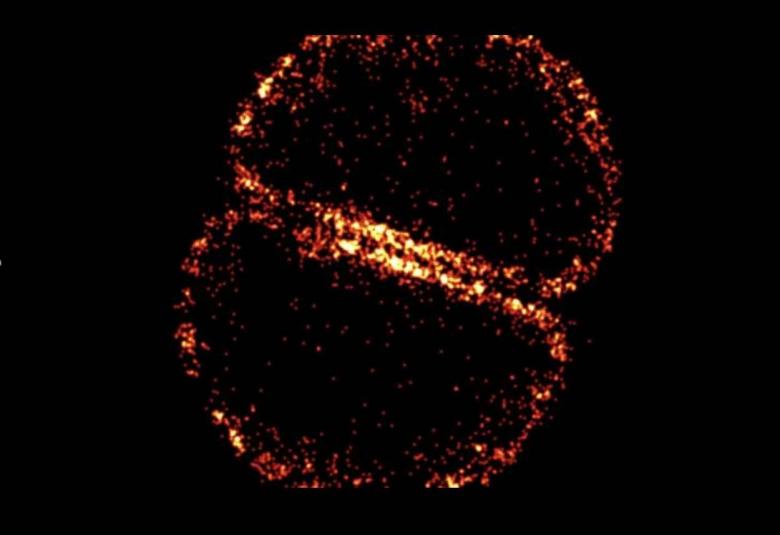
The mechanism which allows β-lactam antibiotics, including penicillin, to kill MRSA has been revealed for the first time.
An international team of researchers led by the University of Sheffield discovered that β-lactam antibiotics kill MRSA (Methicillin Resistant S. aureus) by creating holes in the cell wall which enlarge as the cell grows, eventually killing the bacteria.