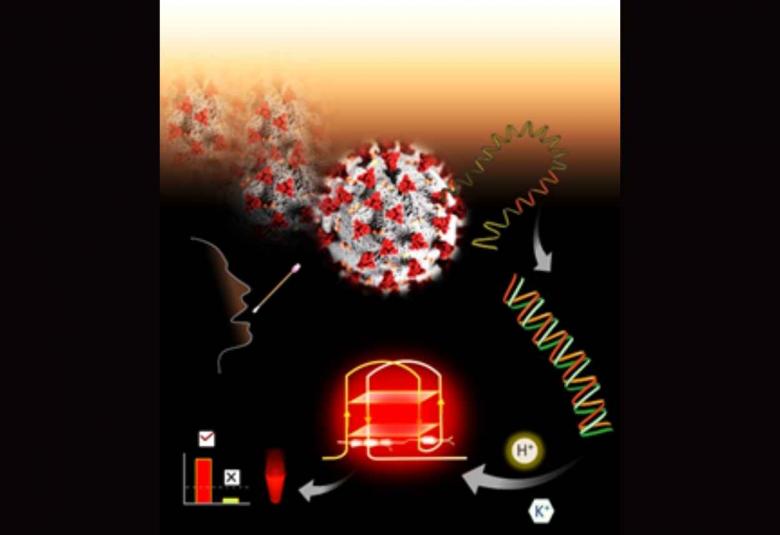
Fluorescence technology to detect new corona virus discovered
A team of scientists has developed a new technology, platform for fluorometric detection of pathogens such as viruses by measurement of fluorescent light emitted. The potential of the new technology has been demonstrated for the detection of SARS-CoV-2. This technology platform can also be used to detect other DNA/RNA pathogens such as HIV, influenza, HCV, Zika, Ebola, bacteria, and other mutating/evolving pathogens.