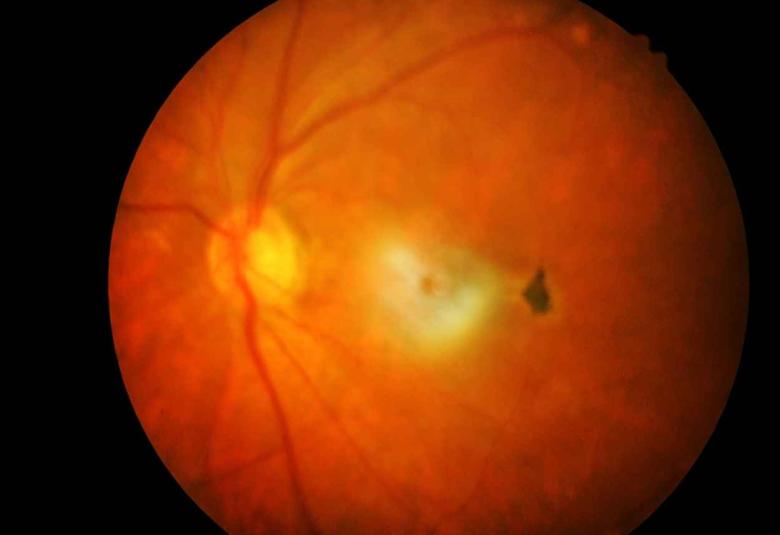
Nanoscope Therapeutics Receives Fast Track Designation by the FDA for MCO-010 for the Treatment of Retinitis Pigmentosa
Nanoscope Therapeutics Inc., a clinical-stage biotechnology company developing gene therapies for retinal degenerative diseases, today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation (FTD) to MCO-010, an ambient-light activatable Multi-Characteristic Opsin (MCO) optogenetic monotherapy to restore vision in blind patients, for the treatment of retinitis pigmentosa (RP) via intravitreal injection.