Q.2. (b) Explain frequency shift due to inductive and mesomeric effects in IR spectroscopy
(b)Frequency shift due to inductive: The inductive effects solely depends upon the ‘intrinsic’ tendency of a substituent to either release or withdraw electrons- by definition, its electronegativity acting either through the molecular chain or through space. This effect usually weakens steadily with increasing distance from the substituent
[adsense:336x280:8701650588]
The inductive effects shall now be discussed specifically with regard to the various functional moieties such as: amides, acyl chlorides, alkyl esters and aryl esters:
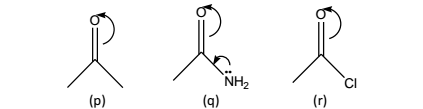
Amides:(q), the +M effect causes weakening of the C=O bond, leading to the corresponding ketone (p). In this particular instance, the –I effect of nitrogen is being dominated by +M effect.
Acyl chlorides: (r), the –I effect of CI is more effective than +M effect, thereby causing an opposite shift (to higher frequency).
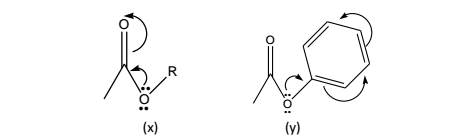
Alkyl esters: (x), it has been observed that a conflict between I and M effects invariably takes place in the case of esters. Here, the non-bonding electrons residing on oxygen enhance the +M conjugation thereby decreasing the C=O frequency.
Aryl esters: (y), Here the non-bonding electrons located on oxygen are partially drawn into the benzene ring and thereafter their conjugation with C=O is minimized. The net effect would be that –I effect of oxygen becomes dominant and consequently C=O moves to a higher frequency.
Field effects: It has been observed that two functional groups often influence each other’s vibrational frequencies by a through-space interaction that may be either steric and/or electrostatic in nature. A typical example of ortho-chlorobenzoic acid esters is shown below:
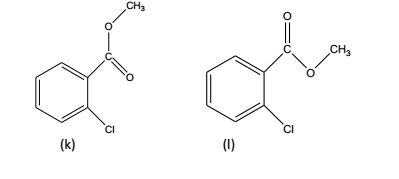
In the above instance, the field effect shifts the C=O frequency in the rotational isomer (k) and not in the isomer (1). As both isomers are usually found to be present together, therefore, two C=O str. absorptions are observed in the spectrum of this compound.
Frequency shift due to Mesomeric (or Resonance) Effect: Whenever a molecule can be represented by two or more structures that differ only in the arrangement of electrons- that is, by structures that have the same arrangement of atomic nuclei- there is resonance. It may be further explained with the help of the following typical examples:
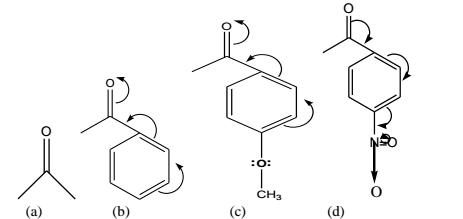
-OMe: Electron Releasing Moiety
-NO2: Electron withdrawing Moiety
In (b) above, the presence of a phenyl ring increases the mesomeric shift thereby lowering C=O str. frequency.
+M Group: p-OMe (an electron releasing function)- its presence as depicted in (c) above will further lower frequencies due to enhanced mesomeric effect.
-M Group: p-NO2 (an electron withdrawing function)- its presence as shown in (d) above will further increase frequencies due to decreased mesomeric effect.









