About Author
Kajal Sahu*
Bhupal Nobles’ College of Pharmacy, BN University, Udaipur (Raj.), 313001
Email : kajalsahu18.ks@gmail.com
ABSTRACT : A transdermal patch is a medicated adhesive patch that is placed on the skin to deliver a specific dose of medication through the skin and into systemic circulation. Often, this promotes healing to an injured area of the body. An advantage of a transdermal drug delivery route over other types of medication delivery such as oral, topical, intravenous, intramuscular, etc. is that the patch provides a controlled release of the medication into the patient, usually through either a porous membrane covering a reservoir of medication or through body heat melting thin layers of medication embedded in the adhesive. Transdermal drug delivery offers controlled release of the drug into the patient, it enables a steady blood level profile, resulting in reduced systemic side effects and, sometimes, improved efficacy over other dosage forms. The main objective of transdermal drug delivery system is to deliver drugs into systemic circulation through skin at predetermined rate with minimal inter and intrapatient variations.
INTRODUCTION
Transdermal drug delivery system is the desirable drug delivery system to control and sustain the drug release via, skin. Control release drug system limits the release of drug and improve the efficiency of the drug, which is relatively fast release system containing the same drug.1 Now a days many drugs are administrated orally, but due to the first pass metabolism in increases the dose and decreases the effects of drug. So, transdermal drug delivery system is design to improve the efficiency and bioavailability of the drug and deceases the number of doses.2 Transdermal drug delivery system is administrated by skin and drug is delivered directly into systemic circulation maintaining continuous efficacy. These systems provide drug systemically at a predictable rate and maintain the rate for extended period of time thus eliminating numerous problems associated with oral products such as reduced bioavailability, enhanced first pass hepatic metabolism, relatively short residence time, dose dumping and dosing inflexibility.3 Physicochemical point of view, an ideal transdermal drug candidate has to meet a number of requirements such as drug is highly lipophilic in nature, melting point of the drug is above 150, molecular weight is above 500 Dalton, log p values 1-5, no local toxicity and irritation to skin.4 Delivery of drugs through the skin for systemic effect, called Transdermal Delivery was first used in 1981, when Ciba-Geigy marketed TransdermV (now marketed as Transdermal scope) for motion sickness.5
ADVANTAGES 4-11
1. Avoid first pass GI and hepatic metabolism.
2. Provide consistent control absorption.
3. Reduces side effects
4. Reduce exposure to undesirable metabolites.
5. Greater patient compliance due to an elimination of multiple dosing.
6. Enhance therapeutic efficiency.
7. Easy to apply and remove.
8. Non-invasive and painless
9. Self-administration.
10. Effective for drug with short biological half-lives and narrow therapeutic window.
11. Easy to remove dosing if adverse reaction occurs.
12. Continuous sustain release.
LIMITATIONS 12-13
1. It cannot administer drug that requires high blood levels.
2. Drug or drug formulation may cause skin irritation and sensitization.
3. The barrier function of the skin changes from one site to another on the same person, from person to person and with age.
4. Not practical, when the drug is extensively metabolized in skin and when molecular size is great enough to prevent the molecules from diffusing through the skin.
5. May cause allergic reaction.
6. Long-time adherence is difficult.
TRANSDERMAL ROUTE AND DRUG DELIVERY BY SKIN
The largest organ:
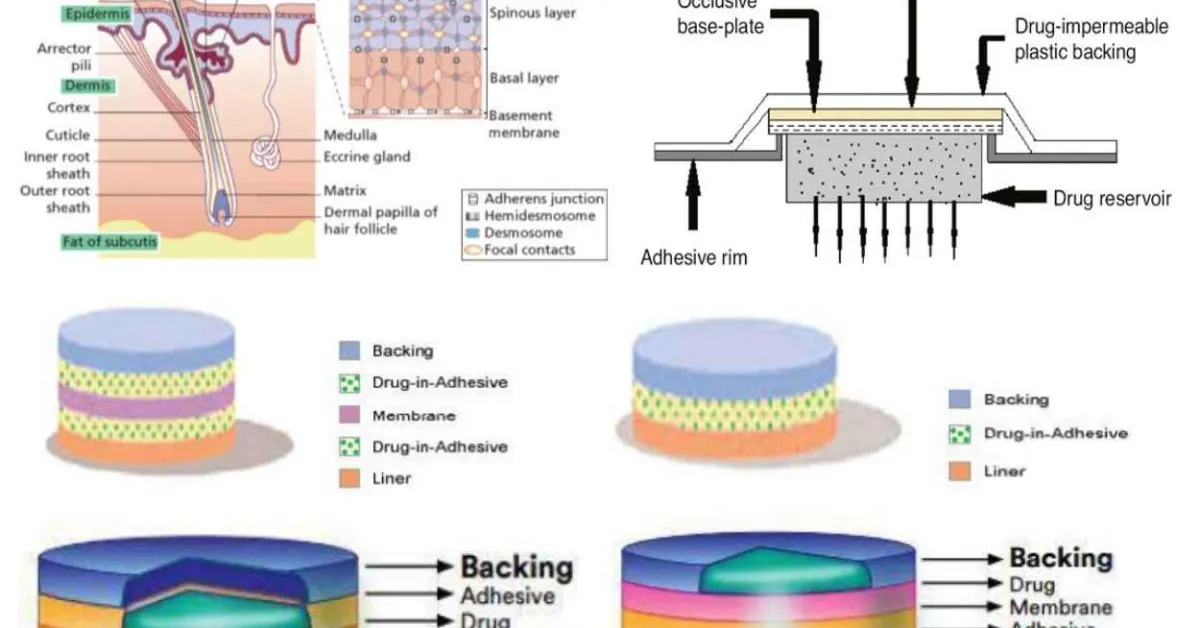
Skin is the largest organ of the human body constituting about 16% of the total body weight. In a healthy adult man, length of skin is 1.5-2 m2 and weighing around 6-10 kg. 14 Skin is made up of different layers of cells; cellular epidermis, underlying dermis and the subcutaneous layer being the major layers Fig 1.1. The rete ridges of the epidermis project down to form the dermis layer. The dermal-epidermal junction provides the mechanical support for the epidermis and acts as a partial barrier against exchange of cells and large molecules. Underlying the dermis is the fatty layer of panniculus adiposus tissues known as the subcutaneous layer. 15 Human skin is of two types viz; glabrous skin (i.e., non-hairy skin) and the hair bearing skin. Glabrous skin is characterized by thick epidermis and presence of sense organs in dermis region. It lacks the hair follicles as well as the sebaceous glands. Glabrous skin is present mainly on palms and soles and has continuous grooved surface with alternating ridges and sulci, forming a unique individual configuration known as dermatoglyphics. Whereas, the hair bearing skin has both the hair follicles as well as the sebaceous glands but lacks the presence of sense organs. 15
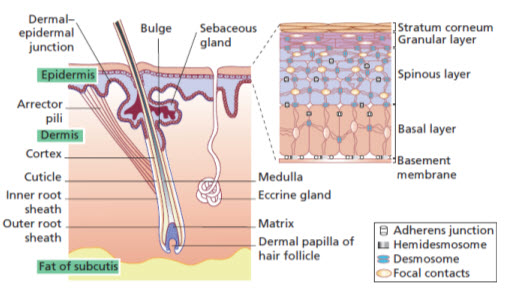
Figure 1: The Skin and Its Appendages 15
Layers of the Skin
1. Epidermis
It is the outermost layer of the skin characterized by presence of stratified squamous epithelium tissues, primarily comprising of keratinocytes in progressive stages of differentiation. 16 Keratinocytes are the building cells of the epidermis. Being avascular in nature, epidermis relies on dermis for nutrient delivery and waste disposal through the basement membrane. 17
Epidermis is divided into four layers; however, the part of the body where the skin is thick, epidermis has five layers. 17,18
i. Stratum Basale (stratum germinativum) – deepest layer, separated from dermis by basement membrane (basal lamina) and attached by hemi-desmosomes. Cells are cuboidal to columnar and are mitotically active stem cells.
ii. Stratum spinosum (prickle cell layer) - irregular, polyhedral cells with processes (“spines”) that extend outward and contact neighbouring cells by desmosomes.
iii. Stratum granulosum - diamond shaped cells which contain keratohyalin granules; aggregates keratin filaments present in cornified cells.
iv. Stratum lucidum - if present, thin clear layer consisting of eleidin (transformation product of keratohyalin); usually seen in thick skin only.
v. Stratum corneum - outermost layer, made up of keratin and horny scales which were once living cells; dead cells known as squamous (anucleate); layer which varies most in thickness, especially thick in callused skin.
Epidermis also contains a variety of cells, major ones being the keratinocytes which makes up about 95% of the epidermal cell population. The other cells include Melanocytes, Langerhans’ cells and Merkel’s cells. 18,19
i. Keratinocytes
• Predominate cell type of epidermis
• Originate in basal layer
• Produce keratin
• Formation of epidermal water barrier
ii. Melanocytes
• Derived from neural crest cells
• Manufacture melanin
• Melanin mainly found in stratum basale and is protective against UV radiation; melanocytes found between cells of stratum basale
• Produced by oxidation of tyrosine to 3,4–DOPA by tyrosinase and then there is the transformation of DOPA into melanin
• Melanin transferred to neighboring keratinocytes by “pigment donation”; involves phagocytosis of tips of melanocyte processes by keratinocytes
iii. Langerhans’ Cells
• Dendritic, antigen-presenting, need special stains to visualize in stratum spinosum (mainly)
• Mesenchymal origin, derived from stem cells of bone marrow – part of mononuclear-phagocytic system
• Birbeck granules
• Express MHC I and MHC II molecules
• Uptake antigens in skin and transport to lymph node
iv. Merkel Cells
• Modified epidermal cells in stratum basale
• Sensory function for fine-touch, most populous in fingertips
• Bound to adjoining keratinocytes by desmosomes and have intermediate keratin filaments
2. Dermis
Dermis is the layer present below the epidermis but is much thicker than the epidermal layer (1-5mm thick). 19 Dermis plays a vital role to sustain and support the epidermis. The connective tissues in the dermis are mainly made of collagen fibres along with some elastin. It is the house to several specialized cells like mast cells and fibroblasts and structures such as blood vessels, lymphatics, sweat glands and nerves. 17 Dermal layer is composed of two main layers of connective tissues- 18
• Papillary layer- it is the thin layer exposed towards the outside comprising of loose connective tissues.
• Reticular layer- it is less cellular, much thicker and deeper layer comprising of dense connective tissue/ bundles of collagen fibers. (Collagen I and III fibers in a ratio of 3:1) 20
These 2 layers are surrounded by a viscous gel that allows lubrication between collagen and elastic fibers, permits nutrients, hormones and waste products to pass through the dermis and provides dermis with bulk allowing it to act as a shock absorber. 21
3. Hypodermis
It is also known as the subcutaneous layer/fat or the Panniculus layer. It is the layer present below the dermis which connects the skin to the underlying fascia (fibrous tissue) of the bones and muscles. Hypodermis is made up of well-vascularized, loose, areolar connective tissues and adipose tissues that act as an energy reserve, insulate the body to prevent heat loss, and serve as a cushion to protect underlying structures from trauma 22 thereby, acting as a shock absorber. It is interlaced with blood vessels and nerves and is the utmost site for storage of fat in the body.
Function of the Skin 23-26
• Protection: Skin is the primary physical barrier that the human body has against the external environment. Skin provides protection against the microorganisms, toxins, dehydration, ultraviolet light, and mechanical damage.
• Sensation: to pain, temperature, touch, and deep pressure.
• Mobility: allowing smooth movement of the body.
• Endocrine activity: The skin initiates the biochemical processes involved in Vitamin D production, which is essential for calcium absorption and normal bone metabolism.
• Exocrine activity: by the release of water, urea, and ammonia. Skin secretes products like sebum, sweat, and pheromones and also exerts important immunologic functions by the secretions of bioactive substances such as cytokines.
• Immunity: development against pathogens.
• Regulation of Temperature: Skin participates in thermal regulation by conserving or releasing heat and helps maintain the body’s water and homeostatic balance.
BASIC COMPONENTS OD TRANSDERMAL DRUG DELIVERY SYSTEM
A. Polymer Matrix :
Polymer is an integral and foremost important component of Transdermal Drug Delivery System. Different classes of polymeric materials have been used to achieve rate-controlled drug delivery. The mechanism of drug release depends upon the physicochemical properties of the dug and polymer used in the manufacture of the device.
The following criteria should be satisfied for a polymer to be used in a transdermal system.
1. Molecular weight, glass transition temperature, chemical functionality of polymer must allow diffusion and release of the specific drug.
2. The polymer should permit the incorporation of a large amount of dmg
3. The polymer should not react, physically or chemically with the drug.
4. They polymer should be easily manufactured and fabricated into the desired product and in expensive.
5. The polymer must be stable and must not decompose in the presence of drug and other excipient used in the formulation, at high humidity conditions, or at body temperature.
6. Polymers and its degradation products must be non-toxic. 27,28
No single material may have all these attributes; certain excipient may be incorporated to alter some properties, for e.g., Cosolvents such as ethanol, propylene glycol, PEG 400 could be added to increase drug solubility. 29
Table 1 : Useful Polymers for Transdermal Devices 27
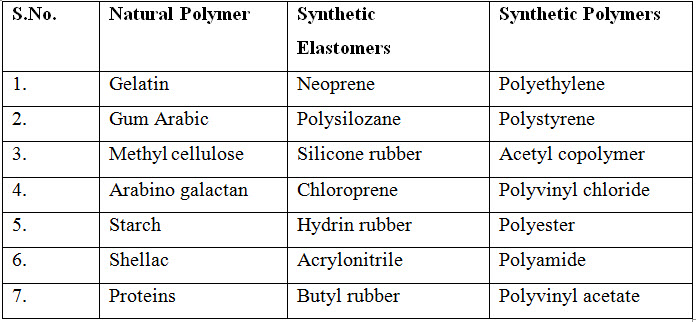
Various techniques have been employed to modify the polymer properties and thus drug release rates 29-31
Cross-linked polymers: The higher the degree of cross linking, the denser the polymer and slower the diffusion of drug molecules through the matrix.
Polymer blends: Polymers have been blended on varying ratios to combine the advantages of the individual polymers. Advantages of polymer blends include easy fabrication of devices, manipulation of drug loading and other devices properties such as hydration, degradation rate and mechanical strength.
Plasticizers: Plasticizers have been known to reduce the stiffness of the polymer backbone, thereby increasing the diffusion characteristics of the drug. Commonly used plasticizers are polyethylene glycol, propylene glycol, glycerol, dibutyl phthalate.
B. Drug Substance : Choice of drug substance is the most important decision in successful development of a Transdermal product. The important drug properties that effect its diffusion through the device as well as through skin are:
Physicochemical properties 32-34
• The drug should have a molecular weight of less than 600 Daltons.
• The log P should be in the range 1-7.
• Melting point should be less than 200 0 C.
• Hydrogen bonding groups should be less than 2.
• It should possess favourable oil: water partition coefficient.
• Drugs highly acidic or alkaline in solution are not suitable for transdermal delivery.
• Solubility in both mineral oil and water should be greater than 1 mg/ml.
• A saturated aqueous solution of the drug should have a value between 5 and 9.
Biological- properties 33,34
• The daily systemic dose should be less than 20 mg.
• The half-life of the drug should be short.
• The drug should not be directly irritant to the skin.
• The drug should not stimulate an immune reaction in the skin.
• Drugs, which degrade in GI tract or are inactivated by hepatic first pass effect, are suitable for transdermal delivery.
• Tolerance to the drug must not develop under the near zero order release profile of transdermal delivery.
• Drugs which have to be administered for a long time or which cause adverse effects to non-target tissues can also be formulated for transdermal delivery.
C. Penetration Enhancers : A problem encountered by formulation scientists in transdermal drug delivery is the ability to attain therapeutic levels of the drug candidate in the systemic circulation. This necessitates the use of enhancers in the development of transdermal formulations. They promote skin permeation and are considered as an integral part of most transdermal formulations. They can modify the skin's barrier to penetration either by interacting with the formulation that is applied or with the skin itself. 29,35
Various criteria that ideal penetration enhancers must meet are:
• Ability to act specifically, reversibly and for predictable duration.
• Pharmacological inertness.
• Nontoxic, non-allergenic, non-irritating.
• Controlled and reverse enhancing action.
• Should not cause loss of body fluids, electrolytes or other endogenous materials.
• Should be a good solvent for drugs.
• Chemical and physical compatibility with drugs and other pharmaceutical excipient with which it is used.
• Rapid onset of action.
• Odourless, colourless and economical and cosmetically acceptable.
• Readily incorporated into the delivery system.
• Following removal of the enhancer, the stratum corneum should immediately and fully recover its normal be barrier property.
• The barrier function of the skin should decrease in one direction only. 36-38
Enhancers increase the penetration of permeants by disrupting the structure of the skin's outer layer "stratum corneum" and increasing penetrant solubility. Disruption either by chemical means, may affect both intracellular and extracellular structure.
D. Other Excipients
Adhesives: The fasting of all transdermal devices to the skin using a pressure sensitive adhesive that can be positioned on the face or in the back of device is necessary.
Both adhesive layers should fulfil the following criteria:
i. Should not cause irritation, sensitization or imbalance in the normal skin flora during
its contact with the skin.
ii. Should adhere to the skin aggressively.
iii. Should be easily removable without leaving an unwashable residue.
The face adhesive system should also fulfil the following criteria:
• Should not affect the permeation of the drug.
• Should allow the delivery of simple absorption enhancers.
• Should not deteriorate the adhesive properties as the drug enhancer and excipients permeate into the adhesive. 39
The three major classes polymers evaluated for potential medical applications in TDDS include:
• Polyisobutylene-type pressure sensitive adhesives
• Acrylic type pressure sensitive adhesives
• Silicone type pressure sensitive adhesives 29

Figure 2: Transdermal Drug Delivery Device 27
Rate controlling membranes : Rate controlling membranes in transdermal devices govern drug release from the dosage from. Membranes made from natural polymeric material such as chitosan show great promise for use as rate controlling membranes. Recently composite poly-2-hydroxyethyl methacrylate (PHEMA) membranes have been evaluated as rate controlling barriers for transdermal application. 28, 39
Backing laminates: The primary function of the backing laminate is to provide support.
Ideal characteristics
• Must be impermeable to drugs and permeation enhancers.
• Should have a low moisture vapour transmission rate.
• Must have optimal elasticity, flexibility, and tensile strength.
• Must be chemically compatible with the drug, enhancer, adhesive and other excipients.
• Must be relatively in expensive.
• Must allow printing and adhesive lamination.
Typical Backing membranes are composed of a pigmented layer, an aluminium vapor coated layer, a plastic film (polyethylene, polyvinyl chloride, polyester) and a heat seal layer. 29
Release liners: The release liner has to be removed before the application of transdermal system, and it prevents the loss of the drug that has migrated into the adhesive layer during storage. It also helps to prevent contamination. It is composed of a base layer, which may be non-occlusive or occlusive, and a release coating layer made of silicon or Teflon. Other materials include polyesters, foil, Mylar and metallized laminate. 29
Four major Transdermal System 38
• Single-layer Drug-in-Adhesive
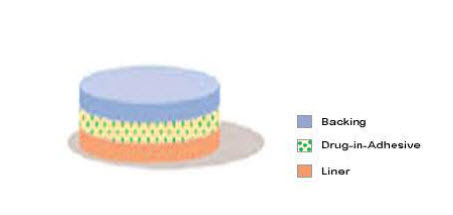
Figure 3: Single layer drug-in-adhesive 38
The Single-layer Drug-in-Adhesive system is characterized by the inclusion of the drug Z within the skin-contacting adhesive. In this transdermal system design, the adhesive not only serves to affix the system to the skin, but also serves as the formulation foundation, containing the drug and all the excipients under a single backing film. Fig 3
• Multi-layer Drug-in-Adhesive
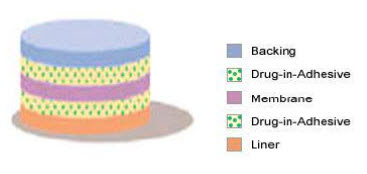
Figure 4: Multi-layer drug-in-adhesive 38
The Multi-layer Drug-in-Adhesive is similar to the Single-layer Drug-in Adhesive in that the drug is incorporated directly into the adhesive. However, the multi-layer encompasses either the addition of a membrane between two distinct drug-in-adhesive layers or the addition of multiple drug-in-adhesive layers under a single backing film. Fig 4
• Reservoir

Figure 5 : Reservoir-drug in-adhesive 38
The Reservoir transdermal system design is characterized by the inclusion of a liquid compartment containing a drug solution or suspension separated from the release liner by a semi-permeable membrane and adhesive. The adhesive component of the product responsible for skin adhesion can either be incorporated as a continuous layer between the membrane and the release liner or in a concentric configuration around the membrane. Fig 5
• Matrix

Figure 6: Matrix drug-in-adhesive 38
The Matrix system design is characterized by the inclusion of a semisolid matrix containing a drug solution or suspension, which is in direct contact with the release liner. The component responsible for skin adhesion is incorporated in an overlay and forms a concentric configuration around the semisolid matrix. Fig 6
FACTORS AFFECTING TRANSDERMAL PERMEABILITY
Table 1.1: Factors affecting Transdermal Permeability 39

APPLICATION 40
• Pain Management
• Hormone replacement therapy
• Contraception
• Antiemetics
• Make Urinary Incontinence
• Cardiovascular
• Central nervous system
EVALUATION PARAMETERS
The prepared transdermal patches were evaluated for various physiochemical parameters like physical examination, thickness, folding endurance, weight variation, tensile strength, moisture uptake, moisture loss and drug content.
• Physical examination 41
Transdermal patches were visually checked for their:
• Colour
• Clarity
• Flexibility
• Homogeneity
• Smoothness
• Thickness 41
The thickness of the patches was measured at five different sites on 3 patches using micrometre and the average was calculated.
• Folding endurance
The folding endurance was measured manually for the prepared patches. It is expressed at the number of times the film is folded at the same place either to break the film or to develop visible cracks. This is important to check the ability of sample to withstand folding. This also gives the indication of brittleness.
Folding endurance of the film determined repeatedly by folding a small strip of film at specific area which is 2 cm x 2 cm (4 cm2) was folded at the same place for many times until a crack was observed and then broke. The value of folding endurance is calculated by number of times the patch could be folded without breaking is note down. [121]
• Weight uniformity 42
As weight variation between the formulated patches can lead to difference in drug content and in-vitro behaviour, a study was carried out by weighing 5 patches in an electronic balance. All the patches were selected randomly and should be uniform in size which is 1 cm x 1 cm. The average weight of a patch and its standard deviation was calculated by using the following formulas.
Average weight of each patch = Total weight of 5 patches/5
Standard deviation = √∑ (x-X)2/ n-1
Where x = weight of individual patch
X = average weight
n = number of patches
• Tensile strength 42
The instrument, which was designed in our laboratory, was used for the measurement of tensile strength. Average weight of three patches was taken as the tensile strength.
A small film strip (4 x 1 cm) was cut on a glass plate with a sharp blade. One end of the fixed between adhesive tapes to give support to the film when placed in the film holder. Another end of the film was fixed between the adhesive tapes with a small pin sandwich between them to keep the strip straight while stretching. A small hole was made in the adhesive tape near the pin in which a hook was inserted. A thread was tied to the hook, passed over the pulley and the small pan attached to the other end to hold the weights. A small pointer was attached to the thread, which travels over the graph paper affixed on the base plate. To determine the tensile strength, the film was pulled by means of pulley system. Weights are gradually added to the pan to increase the pulling force till the film was broken. The elongation was determined by noting the distance travelled by the pointer before break of the film on the graph paper. The weight requires to break the film was noted as break force. Tensile strength was calculated by using the following formula:
Tensile strength = Tensile load at break/a.b. (1+ ΔL/L)
Where a, b, and L are width, thickness, and length of strip respectively, and ΔL is the elongation at break.
Break force = weight requires to break the film (kg)
Elongation at break = IB–IO/IO x 100
Where IO = original length of film
IB = length of film breaks when stress applied.
• Moisture uptake 43
The weighed films kept in a desiccator Fig 5.10 at room temperature for 24hrs was taken out and exposed to 75% relative humidity (a saturated solution of sodium chloride) in a desiccator until a constant weight for the film was calculated as the difference between final and initial weight with respect to initial weight. The percentage moisture uptake was calculated by following formula:
% Moisture uptake = [Final weight - Initial weight / Final weight] x 100
• Moisture loss 43
4 cm2 patches were weight separately and kept in desiccators containing calcium chloride at room temperature for 24 hours Fig 5.10. The patches were weight again after 3 days. The percentage moisture content was calculated by following formula:
% Moisture content = [Initial weight – Final weight / Final weight] x 100
• Drug content 44
A 100 ml graduated flask containing phosphate buffer solution pH 7.4 was used to assess the ATO content. 1 cm2 patch was added to the flask and shaked for 4 hours in a mechanical shaker. The resultant solution was filtered and diluted with phosphate buffer pH 7.4 and analysed for the drug content ATO in UV-spectrophotometer at 246 nm. A blank solution consisting of the placebo patches was used. The average reading of three patches was taken as the content of the drug in one patch.
CONCLUSION
Transdermal delivery offers several advantages over oral routes for controlled drug delivery, viz., avoidance of hepatic first pass metabolism, the ability to control drug delivery for longer time than the GIT transit of oral dosage form, the ability to avoid changing physiological environment and chemical or metabolic degradation, the ability to discontinue administration by removal of the system.
REFERENCES
1. Thombre, A.G. and Cardinal, J.R. Biopolymers for controlled drug delivery. In: Swarbrick, J. and Boylon, J.C. Eds, Encyclopedia of Pharmaceutical Technology. Vol.2, Marcel Dekker Inc.; New York; 1990. pp 61.
2. Kandavilli S, Nair V, Panchagnula R. Polymers in transdermal drug delivery systems. Pharm Technol. 2002;26(5):62–81.
3. Divya A, Rao MK, Gnanprakash K, Sowjanya A, Vidyasagar N, Gobinath M. A review on current scenario of transdermal drug delivery system. Int J Res Pharm Sci. 2012;3(4):494–502.
4. Prausnitz, M. R., Mitragotri, S., and Langer, R. (2004). Current status and future potential of transdermal drug delvivery. Nat. Rev. Drug Discov. 3, 115–124. doi:10.1038/nrd1304.
5. Ghosh, T.K. and Banga, A.K. Methods of enhancement of transdermal drug delivery: Part I. Physical and Biochemical Approaches. Pharmaceutical Technology.1993; 75-78.
6. Chein YW. Transdermal Controlled Systemic Medication. New York and Basel: Marcel Dekker Inc; 1987.
7. Patel D, Chaudhary SA, Parmar B, Bhura N. Transdermal drug delivery system: a review. Pharm Innov. 2012; 1: 66–75.
8. Bowen, A.J., John, V.A., Ramirez, M.E. and Good, W.R. Bioavailability of oestradiol from the Alora (0.1 mg/day) oestradiol matrix transdermal delivery system compared with Estraderm (0.1 mg/day), J. Obstet and Gynecol. 1998; 18(6): 575-580.
9. Dalby, R. Transdermal drug delivery, School of pharmacy, Baltimore.
10. Sharma, S.N. and Popii, H. Transdermal drug delivery system, The Eastern Pharmacist. 1990; 41-42.
11. Davila, G.W., Daugherty, C.A. and Sanders, S.W. A short term multicenter, randomized, double-blind dose titration study of the efficacy and anticholinergic side effects of transdermal compared to immediate release oral oxybutynin treatment of patients with urge urinary incontinence, J. Urol 2001; 166: 140-145.
12. 8. Patel D, Chaudhary SA, Parmar B, Bhura N. Transdermal drug delivery system: a review. The Pharm Innovation. 2012;1(4):66-75.
13. Dhiman S, Thakur GS, Rehni AK. Transdermal patches: a recent approach to new drug delivery system. Int. J Pharmacy Pharm Sci. 2011;3(5):26-34.
14. Ogunleye O. Burns Overview. Physiopedia. 2021. https://www.physio-pedia.com/index.php?title=Burns_Overview&oldid=263666. Accessed on 20th April 2021.
15. World Health Organization. Burns. 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/burns. Accessed on 13th June 2021.
16. Asselin K. National Burn Awareness Weak. Prevention. 2020.
17. Hettiaratchy S, Dziewulski P. ABC of burns: Pathophysiology and Types of Burns. British Medical Journal. 2004; 328: 1427-1429.
18. Yin S. Chemical and Common Burns in Children. Clinical Pediatrics (Phila). May 2017; 56 (5 Suppl): 8S-12S.
19. Gueugniaud PY, Carsin H, Bertin-Maghit M, Petit P. Current Advances in the Initial Management of Major Thermal Burns. Critical Care Medicine. 2000; 26: 848-856.
20. Nguyen CM, Chandler R, Ratanshi I and Logsetty S. In: Jeschke MG, Kamolz LP, Sjöberg F. and Wolf SE. Eds. Handbook of Burns. Vol. 1. Springer. 2020: pp 529–547.
21. Kirkpatrick JJ, Enion DS, Burd DA. Hydrofluoric Acid Burns: A review. Burns. 1995; 21: 483-493.
22. Lee RC. Injury by Electrical Forces: Pathophysiology, Manifestations, and Therapy.Curr. Probl. Surg. 1997: 677-762.
23. Lee JO, Herndon DN. Burns and Radiation Injuries. In: Feliciano DV, Kenneth L, Moore EE, Eds. Trauma. 6th ed. McGraw-Hill; 2008. p. 1051-1066.
24. Jeschke MG, Van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primers. 2020; 6(1): 11.
25. Moore RA, Waheed A, Burns B. Rule of Nines. Stat Pearls (Internet), 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513287/ Accessed 1st July 2021.
26. Murari A, Singh KN. Lund and Browder Chart-Modified Versus Original: A Comparative Study. Acute Crit Care. 2019; 34(4): 276-281.
27. Sharma, S.N. and Popii, H. Transdermal drug delivery system, The Eastern Pharmacist, 1990: 41-42.
28. Kydonieus, A.F. in: Transdermal Delivery of Drugs (Kydonieus, A.F. and Berner, B., eds.), CRC Press, Florida, 1987, 1, p 3.
29. Patani, G.A. and Chien, Y.W. Transdermal drug delivery devices: system design and composition, In: Encyclopedia of Pharmaceutical Technology (Swarbrick, J. and Boylon J.C. eds.), Marcel Dekker Inc., New York, 1999, vol.18, 317-320, 329.
30. Aslani, P. and Kennedy, R.A., J. Contr. Rel. 1996; 42: 75-82.
31. Teijon, J.M., Trigo, R.M., Garcia, O. and Blanco, M.D. Biomat. 1997; 18: 383-388.
32. Finnin, B.C. and Morgan, T.M. Transdermal penetration enhancers: applications, limitations and potential, J. Pharm. Sci. 1999; 88(10): 955.
33. Flynn, G.L. and Stewart, B. Percutaneous drug penetration: choosing candidates for transdermal development. Drug Dev. Res. 1988; 13: 169-185.
34. Guy, R.H., Hadgraft, J. and Bucks. D.A.W. Xenobiotica. 1987; 17: 325.
35. Sinha, V.R., Bindra, S.R. and Nanda, A. Cyclodextrins as skin-penetrati011 enhancers. Pharmaceutical Technology. 2003: 120.
36. sun, Y.M., Huang, J.J., Lin, F.c. and Lac, J.Y. Biomat., 1997; 18: 527-533.
37. Godbey, K.J. Pharm Tech. 1997; 21(10): 98-107.
38. The Pharmaceutical R & D Compendium, CMR. In: Transdermal & Transdermal like Delivery System Opportunities: Today & the Future, (Cleary G.w. eds.), 2002.
39. Govil, S.K. Transdermal drug delivery devices, In: Drug Delivery: Fundamental and Applications Marcel Dekker Inc; New York; 1988. 386-389.
40. Dalby, R., Transdermal drug delivery, School of pharmacy, Baltimore.
41. Saoji SD, Atram SC, Dhore PW, Deole PS, Raut NA, Dave VS. Influence of the Component Excipients on the Quality and Functionality of a Transdermal Film Formulation. AAPS Pharm SciTech. 2015;16(6):1344-1356.
42. Alagusundaram M, Chetty CM, Dhachinamoorthi D. Development and evaluation of novel-trans-buccoadhesive films of Famotidine. Journal of Advanced Pharmaceutical Technology & Research. 2011; 2(1): 17-23.
43. Khan FN, Dehghan MHG. Enhanced Bioavailability of Atorvastatin Calcium from Stabilized Gastric Resident Formulation. AAPS Pharm SciTech. 2011;12(4): 1077-1086.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT admin@pharmatutor.org
FIND OUT MORE ARTICLES AT OUR DATABASE