{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Vipul Gandhi*, Sushil Burle, Satish Kosalge
Hi-Tech College of Pharmacy Chandrapur
Padoli Phata, Nagpur Highway, Morwa, Chandrapur-442406.
*vipul96gandhi@gmail.com
ABSTRACT
Some of the problems with Un productive relaxing treatments and insufficient repair aptitude in the central nervous system are most troubling problems for few neurological diseases. Providentially, the development of clinically relevant populations of stem cells has provided an opportunity to overcome the failure of endogenous repair systems and substitute new cells into the injured brain. However, there are still several existing difficulties in interpreting into clinical application. In this review, we mainly focus on the stem cell based therapies for Parkinson’s disease and discuss the possible advantages and drawbacks. We hope this review may provide suggestions for viable policies to overcome the current technical and biological issues relatedto the application of stem cells in Parkinson’s disease (Fu et al., 2015).
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-2587
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 6, Issue 6 Received On: 12/03/2018; Accepted On: 17/04/2018; Published On: 01/06/2018 How to cite this article: Gandhi V, Burle S, Kosalge S; Stem Cell Therapy for Parkinson’s disease: A Review; PharmaTutor; 2018; 6(6); 1-8; http://dx.doi.org/10.29161/PT.v6.i6.2018.1 |
INTRODUCTION:
The Stem cells have the potential to develop into many different types of cells in the body. It mostly serves as a repair system for the body. This type of cells is of two type embryonic cells and adult cells.On a division, the stem cells have a potential while repairing it remains same or develop into another type of cell with more sophisticated functions it mostly develops as a muscle, brain or RBC cells.
The stem cells consist of many different types of cells it many modifies the embryonic and adult cell used to differentiate stem cells by the developmental stage of the animal from where this cellscome. The adult stem cells more correctly termed as “somatic” stem cells meaning from the body mostly found in the fetus, umbilical cord blood, placenta, and infants. In the 3- to 5-day-old embryo, called a blastocyst, the inner cells give rise to the entire body of the organism, including all of the many specialized cell types and organs such as the heart, lung, skin, sperm, eggs and other tissues. In some adult tissues, such as bone marrow, muscle, and brain, discrete populations of adult stem cells generate replacements for cells that are lost through normal wear and tear, injury, or disease. In laboratory scientist and researchers are continuously working on various aspects of stem cell how they are different from other cells, there essential properties and what makes them different from specialized cell types. Many new researchers are also using the stem cell for the screening of new Drugs moiety apart from this it also helpful for the development of model systems to study normal growth and identification of causes of birth defects (Canet et al., 2014).
CLASSIFICATION OF STEM CELLS (Canet et al., 2014):
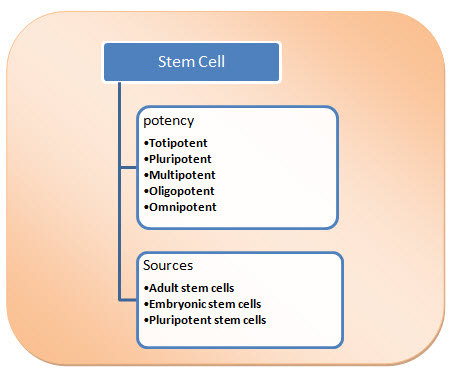
Fig.1. Classification of Stem Cell
ON THE BASIS OF POTENCY
The stem cell classification on the basis of potency.
Totipotent: This type of cells are having the ability to differentiate into all possible cell types. Examples are the zygote formed at egg fertilization and the first few cells that result from the division of the zygote.
Pluripotent: The ability to differentiate into almost all cell types. Examples include embryonic stem cells and cells that are derived from the mesoderm, endoderm, and ectoderm germ layers that are formed in the beginning stages of embryonic stem cell differentiation.
Multipotent: The ability to differentiate into a closely related family of cells. Examples include hematopoietic (adult) stem cells that can become red and white blood cells or platelets.
Oligopotent: The ability to differentiate into a few cells. Examples include (adult) lymphoid or myeloid stem cells.
Omnipotent: The ability to only produce cells of their own type, but have the property of self-renewal required to be labeled a stem cell. Examples include (adult) muscle stem cells.
ON THE BASIS OF SOURCES
The easiest way to categorize stem cells is by dividing them into two types: Early or embryonic and mature or adult. Early stem cells, often called embryonic stem cells, are found in the inner cell mass of a blastocyst after approximately five days of development.
Embryonic stem cells: Embryonic stem cells are self-replicating pluripotent cells that are potentially immortal. They are derived from embryos at a developmental stage before the time of implantation would normally occur in the uterus. The embryos from which human embryonic stem cells are derived are typically four or five days old and are a hollow microscopic ball of cells called the blastocyst.
Adult stem cells: Adult stem cells are undifferentiated totipotent or multipotent cells, found throughout the body after embryonic development that multiplies by cell division to replenish dying cells and regenerate damaged tissues.
Pluripotent stem cells: nowadays the third type of stem cell, with properties similar to embryonic stem cells, has emerged. Scientists have engineered these induced pluripotent stem cells (is cells) by manipulating the expression of certain genes - 'reprogramming' somatic cells back to a pluripotent state.
WHAT IS PARKINSON’S DISEASE?
Parkinson’s disease is a type of disorder of movements that can affect the ability to perform common and daily activities. As this disease is linked to a wide range of symptoms, These symptoms are typically divided into; i) Motor symptoms and ii) Non-Motor symptoms.
The most common motor symptoms of Parkinson's disease are tremor (a form of rhythmic shaking), stiffness or rigidity of the muscles, and slowness of movement ( bradykinesia). A person with Parkinson's disease may also have trouble with balance, posture, coordination, and walking. Common non-motor symptoms of Parkinson's disease are constipation, sleep problems, depression, anxiety, and fatigue (Sung et al., 2017).
CAUSES OF PARKINSONS DISEASES
Genetic Factors: Scientists have estimated that less than 10% of cases of Parkinson's disease are primarily due to genetic factors. The most usual genetic effect that triggers Parkinson's disease is a mutation in a gene called LRRK2. This defect is particularly found in families of North African or Jewish descent. Mutations in alpha-synuclein have been found to trigger Parkinsons disease, but these are very few. In most cases of Parkinson's disease, no primary genetic cause can be found.
Environmental Factors: Certain environmental effects, such as exposure to chemicals, pesticides or certain heavy metals and repeated head injuries, can increase the risk of Parkinson's disease. It may not affect in certain cases due to habituation of environment. However, the environmental factors do influence the development of Parkinson's disease, perhaps particularly in people who also have a genetic susceptibility.
Other Factors: This factor includes age because of it most common in adults above the age of 50. Mostly the women are having more percentage than men. Parkinson's disease often seems to affect Caucasians more than African Americans or Asians. Now that you know a bit more about what Parkinson's disease actually is, the following chapter will provide greater detail about what to expect in terms of symptoms (Khan et al., 2013).
MOTOR AND RELATED SYMPTOMS OF PARKINSON'S DISEASE:
There are five primary motor symptoms of Parkinson's disease:
- Tremor
- Rigidity
- Bradykinesia (slow movement)
- Postural instability (balance problems)
- Walking/gait problems.
NON-MOTOR SYMPTOMS OF PARKINSON'S DISEASE
- Disturbances in the Sense of Smell
- Sleep Problems
- Depression and anxiety
- Fatigue
- Mental processes
- Weight loss
- GIT related issues
- Lightheadedness
- Urinary issues
- Sexual concern
- Sweating
- Melanoma
STAGES OF PARKINSON’S DISEASE:
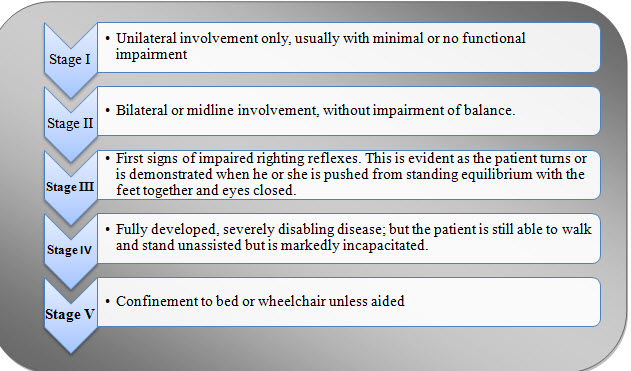
Fig.2. Stages of Parkinson’s disease
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PATHOPHYSIOLOGY
The characteristic pathological features of Parkinson's disease are a reduction in neurons of pigmented brainstem nuclei, along with the presence of eosinophilic inclusion bodies, called Lewy bodies, in living cells. In Parkinson's disease,before the appearance of symptoms, there is a loss of over 80% of nigral neurons. The ‘Braak hypothesis’ has been proposed to account for the spread of pathology within the Parkinsonian brain and suggests that α-synuclein may first accumulate in the lower brainstem and then gradually arises rostrally to affect critical brain regions including the substantia nigra and ultimately the cerebral cortex (Braak et al., 2003). Dopaminergic neurons are not only cells to die within the brainstem, but an excess of other nuclei and neurotransmitter systems are involved too. For example, cholinergic neurons within the pedunculopontine nucleus degenerate, providing potential clinicopathological correlates with postural instability, swallowing difficulty (dysphagia) and sleep disturbance (REM sleep behavioral disturbance). The involvement of this nucleus in Parkinson's disease may explain why dopaminergic therapy is relatively ineffective in treating these particular clinical problems. Within the striatum, changes occur within γ-aminobutyric acid-containing neurons, as a concern of nigrostriatal dopaminergic deficiency and also an anon-physiological dopaminergic replacement. These changes are thought to play a key role in mediating the development of involuntary movements (dyskinesias) which develop after a number of years of levodopa treatment. The loss of noradrenergic and serotonergic neurons within the locus coeruleus and the raphe nucleus, correspondingly, may provide a pathophysiological basis for depression, which is common in Parkinson's disease.
STEM CELL THERAPY:
The stem cell is the techniques most useful for cure of injury or treats the disease. This type of cell is having the ability to self-renew which offers significant potential for generation of tissue that can potentially replace diseased and damaged area in the body with minimum risk of rejection and side effects. The medical researcher anticipate that adult and embryonic stem cell will soon be able to treat cancer,diabetes ,parkinsonison’s, Huntington's disease, etc. (Kalra and Tomar 2014)
Treatment of Parkinson’s by stem cell therapy:
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, which affect 1 % of the population worldwide after the period of 65 years of age. The typical symptoms of Parkinson’s disease are bradykinesia, rigidity and resting tremor(Tanner and Goldman 1996). The main pathological features are extensive loss of dopamine neurons in the Substantia Nigra pars compacta and the accumulations of cytoplasmic eosinophilic inclusions, Lewy bodies (Forno 1996). The cause of degenerated nigrostriatal dopaminergic neurons is unknown. Presently the therapy given to Parkinson’s patients include levodopa, monoamine oxidase inhibitors, dopaminergic agonists, and deep brain stimulation. Normally, the effectiveness of oral medications starts to wear off after a period of 5 years (Jankovic 2005). Moreover, these treatments cannot repair the damaged dopaminergic striatal projections; therefore, restorative approaches should be considered in order to improve the therapeutic action. Since Parkinson’s disease patients display selective degeneration of SN dopaminergic neurons, cell replacement therapies which can produce functional dopaminergic neurons may be a valuable therapeutic tactic. To achieve a successful cell-based therapy in Parkinson’s disease, some criteria for cell transplantation are generally suggested (Lindvall and Hagell 2000; Lindvall and Kokaia 2006; Lindvall et al. 2004).
The cells should possess the molecular, morphological and electrophysiological properties of dopaminergic neurons in substantia nigra;
The therapy should enable 100,000 or more DA neurons to survive long-term in human putamen;
The grafted cells should re-establish a dense terminal network throughout the striatum to functionally integrate into host neural circuitries. Here we review the progress of stem cell therapies and discuss the major problems encountered in Parkinson’s disease.
Graft (Implantation):
The content of graft is the serious issue when performing the transplantation. It is presently unknown whether symptomatic relief would be best achieved by implanting of DA neurons or a graft containing a portion of glial cells. During embryonic development,several studies support the necessary role of astrocytes in neural differentiation, implying that glial cells are important for fate determination of precursors during implantation (Song et al. 2002). So, mesencephalic tissues consisting glial cells were most often used in previous studies. Another key issue in performing grafts for PD treatment is that implanting the most suitable subtype of DA neurons is also critical for the result of transplantation. DA rich-ventral mesencephalic grafts contain two types of DA neuron progenitors, containing A9 Substantia Nigra neurons and A10 dopamine neurons of the Ventral Tegmental Area. The A9 subtype DA neurons only sends innervated axons into the striatum in rats suggesting that mesencephalic grafts with more A9 subtype DA neurons would be more beneficial for PD treatment. In the late 1980s, clinicians transplanted human embryonic or fetal ventral mesencephalic tissues into PD patients, but the results were varied. In Madrazo and Lindvall’s open-label trials, PD patients showed improvement of Unified Parkinson’s Disease Rating Scale (UPDRS) after receiving fetal DA neuron graft (Madrazo et al. 1988; Lindvallet al. 1989). Yet, the results from two double-blind trials funded by the National Institutes of Health (NIH) in the 1990s showed no major effects (Freed et al. 2001; Olanow et al. 2003). Even more, several side-effects have been shown in PD patients who received these transplantations. The results of these two open-label and double-blind trials raise critical issues regarding ethical considerations and may enhance controversy which can dissuade the potential use of transplants for PD.
ES cell‑derived DA neurons ES cells are one important source that has been used to differentiate into DA neurons in the laboratory. DA neurons derived from rodents and humans have been shown to survive and function after transplantation into the striatum of PD rats (Kim et al. 2002; Yang et al. 2008). Furthermore, the uptake of [18F]-DOPA also increased 14 weeks after transplantation, suggesting exogenous ES cell-derived DA neurons could offer the functional recovery of DA neurons. Until now, ES cells are still the most promising source to differentiate into DA neurons (Kim et al. 2002; Rodriguez Gomez et al. 2007); however, the efficiency to differentiate into DA neurons and the existence rate of these neurons after transplantation is still low. For example, prior reports showed less than 300 tyrosine hydroxylase (TH)-positive neurons survived after transplanting 100,000–400,000 ES cells into the striatum (Brederlau et al. 2006; Ben-Hur et al. 2004). Therefore a critical issue that must be resolved to enhance recovery after transplantation in PD is an improvement of the differentiation and survival rate (Bergman and Deuschl 2002)
iPSC-derived DA neurons:
Subsequently, the iPSC technique was recognized in 2006, another cell source to generate DA neurons was provided. At first, in 2008, the DA neurons were made from mouse into the striatum of a rat Parkinson’s Disease model, thereby easing the symptoms of Parkinson’s Disease. In 2010, DA neurons differentiated from ipscsofParkinson’s Disease patients were transplanted into Parkinson’s Disease transgenic rats, and these neurons survived for some months and further eased the symptoms of Parkinson’s Disease(Hargus et al. 2010). These transplanted cells did not display α-synuclein positive inserts bodies in hosts, signifying that in Parkinson’s Disease patients may be a viable option. However, several reports have mentioned patient-derived ips are still more vulnerable to Parkinson’s Disease because of mutations or epigenetic markers in cells (Badger et al. 2014; Beevers et al. 2013; Sanchez-Danes et al. 2012). Furthermore, the risk of tumor formation still needs to be reduced before the medical application can be understood (Petit et al. 2014). Since the method of iPSC production was only developed a few years ago, further optimization may overcome these problems.
Nscsand NSC derived DA neurons nscsare multipotent stem cells that are defined as “neurally” designed cells and retain their regional specificity (Horiguchi et al. 2004). Therefore, NPCs that are derived from a chiefly DA vicinity, such as ventral mesencephalon (VM), should be the most suitable source of DA neurons. Several reports have shown the potential skill of nscsto differentiate into DA neurons (Tan et al. 2014, 2015), and also established the advances of symptoms in Parkinson’s Disease models after transplantation of NSC-derived DA neurons (Parish et al. 2008; Redmond et al. 2007). Furthermore, overexpression of different genetic factor, such as Lmx1a, glial cell line-derived neurotrophic factor (GDNF), Brn4 and TH, in nscshasimproved the valuable effects of NSC-derived DA neurons (Tan et al. 2014; Wu et al. 2015; Wakeman et al. 2014). In animal studies, NSC-derived DA neurons overexpressing Nurr1, a critical factor involved in DA requirement and survival, has led to functional improvement in rats treated with 6-hydroxydopamine (6-OHDA), a toxic-induced Parkinson’disease model (Park et al. 2006). However, the survival rate of TH positive neurons after a replacement was less than 4.3 % (Park et al. 2006; Studer et al. 1998). In 2008, Parish et al. Transfected Wnt5a into nscs from mouse VM, generating tenfold more DA neurons with TH positive signal than the conventional FGF2-treated nscs from VM and causing a functional recovery in 6-OHDA mice (Parish et al. 2008). Aside from VM, investigators have also derived nscs from the SVZ, which is another well-known source for these cells. The auxiliary of nscs from SVZ boosted the recovery of Parkinson’s Disease symptoms, but the survival rate of these cells was still low (Meissner et al. 2005; Richardson et al. 2005). Therefore, the most important matter that must be overcome in order to achieve NSCrelocation is to increase the cell number and existence rate of transplanted cells.
IMMUNE RESPONSE:
Although the brain is considered as an immune-privileged site, the host immune system still responds to the grafts. The interaction between implantation and the endogenous immune system affects the survival of grafted cells. In several clinical trials, transplantation without adequate immune-suppression may have led to poor outcomes (Freed et al. 2001; Olanow et al. 2003), while transplantation with an immune-suppressant, such as cyclosporine, azathioprine,and prednisolone, produced better effects (Lindvall et al. 1989). Unfortunately, patient symptoms worsened after withdrawal of immune-suppression, and postmortem showed grafts were surrounded by activated microglia and immune reactivity (Olanow et al. 2003). These results imply that immune reactions exert a negative effect during transplantation, and it would be necessary to use an immunosuppressant in combination with grafting; however, further studies are still needed to determine the optimal immunosuppressant and the period of treatment.
MAJOR DISPUTES AFTER GRAFTS:
Depending on the reports of several cell-based studies in both animals and humans, there are two major apprehensions related to grafts.
Graft Induced Dyskinesia (GID)
The occurrence of dyskinesia after transplantation was first reported by Defer et al. (1996), but did not obtain much courtesy till Freed’s trial in 2001 (Freed et al. 2001). They described the development of “graft persuaded dyskinesia (GID)” in 15 % of transplanted patients 1 year after transplantation. This surprising symptom reached 56 % in Olanow’s study (Olanow et al. 2003). It is speculated that the number of grafted cells used in the surgery-controlled clinical trials was less than those of other more successful studies (Lindvall et al. 1989; Freed et al. 2001). Furthermore, immune-suppression may be also an important aspect since dyskinesia did not develop till immunosuppression withdrawal in several reports (Olanow et al. 2003; Piccini et al. 2005; Lane et al. 2008). The other possible dispute is that other varied grafts were originated in ventral putamen, containing serotonergic neurons. These grafts led to islands of reinnervation and abnormal production of DA (Ma et al. 2002; Carlsson et al. 2009). Hence, transplanting a sufficient number of cells having a pure population of DA neurons in basal ganglion with immunosuppression is suggested to avoid the progress of GID.
Grafts affected by PD process:
Indications that PD pathology may propagate from host to grafts is emerging (Kordower et al. 2008a, b; Li et al. 2008). The presence of LBs and Lewy neurites in grafted DA neurons were generally observed 11–16 years after human fetal mesencephalic transplantation (Kordower et al. 2008a, b; Li et al. 2008; however,α-synucleinstaining is generally not detectable in adults younger than 20-year-old (the Chu and Kordower 2010). These observations imply that PD pathology can be transferred from host to graft (Visanji et al. 2013). The exact reasons for this negative outcome remain unresolved. A recent report showed that fetal cell transplantation in two PD patients remains highly functional even 15–18 years after surgery (Kefalopoulou et al. 2014). Therefore, although the spread of PD pathology may occur after transplantation, the period of beneficial effects from transplantation is still longer than that of current medications, suggesting stem cells still could be a potential clinical therapy (Rana, 2011).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
MECHANISMS OF STEM CELL THERAPY IN PARKINSON'S DISEASE:
The mechanism is classified in two ways it includes:
Direct Repair Pathway. 2. Indirect Repair Pathway
Direct repair pathway:
It includes supplementing endogenous neurogenesis, DA neuron differentiation (Park et al. 2012), DA release (Rodriguez-Gomez et al. 2007; Bouchez et al. 2008), striatum reinnervation (Kordower et al. 1995) and neural trails integration (Piccini et al. 2000; Bjorklund et al. 2002).
Indirect repair system through trophic factors: Stem cells express various neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), cerebral dopamine neurotrophic factor (CDNF), nerve growth factor (NGF), or glial-derived neurotrophic factor (GDNF), and facilitate DA neuronal differentiation and maintenance. These passerby effects are mainly likely to result from grafts comprised of NSCs and MSCs (Rafuse et al. 2005; Tolar et al. 2010; Yasuhara et al. 2006; Lu et al. 2003). However, it is still hard to distinguish clearly which pathway shows a dominant role, and, as a result, it is generally assumed that both direct and indirect pathways contribute to the beneficial effects after transplantation
Treatment Efficacy from Patient Perspective:
We need to detect the outcome measures that are meaningful not only to investigators and clinicians but also to patients and for that we will work in partnership with end users in order to safeguard our key outcomes which are related to patient perspective. As a mean, we will early on establish a “user board”. An ultimate challenge is also to explicitly identify/develop tools that imitate real-life challenges (e.g. outdoor mobility) in standardized settings to certify that potential treatment effects translate into “real-world” settings. Pilot tests are then needed well advance of a clinical trial in order to determine the acceptability, probability,and reliability of such assessments. Toobin-depth investigate the patient viewpoint, qualitative methods (e.g. interviews) will be used as a complement to quantitative methods that include both unbiased measures and patient-reported outcomes measures. Taken together, our measurements will hit the difficulty of health condition such as PD by covering both functions, activities of importance in daily life and participation, i.e. “involvement in life situations”(International Classification of Functioning, Disability, and Health, ICF, WHO, 2001)
CONCLUSION:
From the above theories, it has been concluded that there is still no perfect treatment for Parkinson'sdiseasehas been developing yet. however, the outcomes from the clinical trials have not been proven consistent or convincing. This may be due to a combination of factors, such as patient selection, amount and mode of tissue engraftment and the level of immune-suppression. Moreover, another side effect to be considered is GID. Fortunately, grafted tissues were unaffected by PD progression within 10 years after transplantation, so the treatment of PD with stem cell grafts is still a favorable direction. The major advantage of this strategy is the restorative and trophic abilities of the grafted cells which reach far away from drugs prescribed in a recent practice.
Another thing is the regeneration of dopaminergic neurons and the maintenance of dopamine homeostasis which are important for successfully treating this disease as the patients with PD are greatly affected by the loss of dopaminergic neuron. However, the major problem to developing therapeutic strategies for neurodegenerative diseases is the brain complexity. Due to the reason that the transplanted stem cells can redevelop neurons and glia in an optimal microenvironment and produce neuroprotective molecules, the stem cell therapy has great potential to treat PD as well as other neurodegenerative diseases.
REFERENCES:
1. Ann Neurol 1994;36: 348–355. 35 Collins LM, Toulouse A, Connor TJ et al. Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology 2012;62:2154– 2168.
2. Arrasate M, Mitra S, Schweitzer ES et al. Inclusion body formation reduces levels of mutant hunting in and the risk of neuronal death. Nature 2004;431:805–810.
3. Backlund E.O, Granberg P.O, Hamberger B. et al. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J Neurosurg 1985;62:169–173.
4. Barker R.A, Ouellet J.D., Parmar M., “Cell based therapies for Parkinson disease —past insights and future potential”, Neurology Volume 11, 2015.
5. Bergman H. and Deuschl G., “Pathophysiology of Parkinson’s Disease: From Clinical Neurology to Neuroscience and Back”, The Hebrew University, Jerusalam, Israel, Department of Neurology, Christian Alberechts Univesistst Kiel, Germany, vol. 17, 2002.
6. Bodner RA, Outeiro TF, Altmann S et al. Pharmacological promotion of inclusion formation: A therapeutic approach for Huntington’s and Parkinson’s diseases. Proc Natl Acad Sci USA 2006;103:4246–4251.
7. Canet R., Geoffrey P. Lomax, Ellen G. Feigal, Catherine Priest, “Proceedings: Cell Therapies for Parkinson’s disease From Discovery to Clinic”, California Institute for Regenerative Medicine, San Francisco, California, USA 2014.
8. Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat Neurosci 2005;8: 657–663.
9. Cooper O, Seo H, Andrabi Setal. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci Transl Med 2012; 4:141ra90.
10. Correia A.S, Anisimov S.V, Li J.Y &Brundin P., “Stem cell-based therapy for Parkinson’s disease”, Annals of Medicine. 2005; 37: 487–498.
11. de la Fuente-Fernandez R, Schulzer M, Mak E et al. The role of the Lewy body in idiopathic Parkinsonism. Parkinsonism Relat Disord 1998;4:73–77.
12. Dexter DT, Wells FR, Lees AJ et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson’s disease. J Neurochem 1989;52:1830– 1836.
13. Do CB, Tung JY, Dorfman E et al. Webbased genome-wide association study identifies two novel loci and a substantial genetic component for Parkinson’s disease. PLoS Genet 2011;7:e1002141.
14. Fu M.H, Li C.L, Lin H.L, Chen P.C., Calkins M.J., Chang F.L., Cheng P.H and Yang H.S, “Stem cell transplantation therapy in Parkinson’s disease”, Department of Physiology, College of Medicine, National Cheng Kung University, Tainan 70101, Taiwan, 2015.
15. Jankovic J, Poewe W. Therapies in Parkinson’s disease. Curr Opin Neurol 2012;25:433– 447.
16. JuckerM,Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol 2011;70: 532–540.
17. K. Kalra and P.C. Tomar, “Stem Cell: Basics , Classification and Application”, American Journal of Phytomedicine and Clinical Therapeutics IISN 2321 – 2748
18. Keller MF, Saad M, Bras J et al. Using genome-wide complex trait analysis quantify ‘missing heritability’ in Parkinson’s disease. Hum Mol Genet 2012;21:4996–5009.
19. Khan F.F, Tanveer T., Gul A., “Parkinson disease and use of stem cells for therapeutic approaches of Parkinson disease”, IOSR Journal of Pharmacy and Biological Sciences (IOSR-JPBS) e-ISSN: 2278-3008, p-ISSN:2319-7676. Volume 7, Issue 5 (Sep. – Oct. 2013), PP 73-82
20. Krack P, Limousin P, Benabid AL et al. Chronic stimulation of subthalamic nucleus improves levodopa-induced dyskinesias in Parkinson’s disease. Lancet 1997;350:1676.
21. Lindvall O, Backlund EO, Farde L et al. Transplantation in Parkinson’s disease: Two cases of adrenal medullary grafts to the putamen. Ann Neurol 1987;22:457–468.
22. Lindvall O. &Kokaia Z., “Stem cells for the treatment of neurological disorders”, Laboratory of Neurogenesis and Cell Therapy, Nature Publishing Group © 2006
23. LindvallO.Developing dopaminergic cell therapy for Parkinson’s disease—give up or move forward? Mov Disord 2013;28:268–273.
24. Marquardt L.M &Heilshorn S.C, “Design of Injectable Materials to Improve Stem Cell Transplantation”, Springer International Publishing AG 2016.
25. Miocinovic S, Somayajula S, Chitnis S et al. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol 2013; 70:163–171.
26. National Collaborating Centre for Chronic Conditions Parkinson’s Disease. National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. NICE Clinical Guidelines, No. 35. London, U.K.: Royal College of Physicians, 2006.
27. Nguyen HN, Byers B, Cord B et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 2011;8:267–280.
28. Okun MS. Deep-brain stimulation for Parkinson’s disease. N Engl J Med 2012;367: 1529–1538.
29. Parsons TD, Rogers SA, Braaten AJ et al. Cognitive sequelae of subthalamic nucleus deep brain stimulation in Parkinson’s disease: A meta-analysis. Lancet Neurol 2006;5:578– 588.
30. Politis M, Wu K, Loane C et al. Serotonin neuron loss and non motor symptom continue in Parkinson’s patients treated with dopamine grafts. Sci Transl Med 2012;4:128ra41.
31. Politis M, Wu K, Molloy S et al. Parkinson’s disease symptoms: Thepatient’s perspective. Mov Disord 2010;25:1646–1651.
32. Rana A.Q, “Etiology and Pathophysiology of Parkinson’s Disease”, Janeza Tridine, Croatia 2011.
33. S´anchez S., Schulte J., Bras C., Metal J., Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet 2009;41:1308–1312.
34. Sayre LM. Biochemical mechanism of action of the dopaminergic neurotoxin 1-methyl4-phenyl-1,2,3,6-tetrahydropyridine(MPTP). ToxicolLett 1989;48:121–149.
35. Shulman JM, De Jager PL, Feany MB. Parkinson’s disease: Genetics and pathogenesis. Annu Rev Pathol 2011;6:193–222.
36. Sian J, Dexter DT, Lees AJ et al. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders
37. Singleton A.B, Farrer M.J, Bonifati V. The genetics of Parkinson’s disease: Progress and therapeutic implications. Mov Disord 2013;28: 14–23.
38. Skibinski G, Finkbeiner S. Longitudinal measures of proteostasis in live neurons: Features that determine fate in models of neuro degenerative disease. FEBS Lett 2013;587:1139– 1146.
39. Standaert D.G , Saint-Hilaire M.H, Thomas C., “Parkinson’s Disease Handbook”, American Parkinson’s Disease Association. 9. K. Takahashi, S. Yamanaka, "Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors". Cell, 2006, 126 (4): 663–76.
40. Su P., Loane C. and Politis M., “The Use of Stem Cells in the Treatment of Parkinson’s Disease”, MariosPolitis, Hammersmith Hospital, Du Cane Road, London W12 0NN, UK;
41. Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov Disord 2013;28:715–724.
42. SulzerD, ZeccaL. Intraneuronal dopamine quinine synthesis: Areview. Neurotox Res 2000; 1:181–195.
43. Sung S C., Gi H.R., Da H K., Lee DS, Lee S., Kim SY,., Kwang H L, Woo y.k., Yun S. S., Gi S.I., Hu T., Lee H.J.,Stem Cell Applications in Parkinson’s Disease, (2017), J Alzheimers Dis Parkinsonism, 2017, Vol.7.
44. TemelY.Limbiceffectsofhigh-frequency stimulation of the subthalamic nucleus. In:Gerald L, ed: Vitamins and Hormones. Waltham, MA: Academic Press, 2010:47–63.
45. Weiss HD, Marsh L. Impulse control disorders and compulsive behaviors associated with dopaminergic therapies in Parkinson disease. NeurolClinPract 2012;2:267–274.
46. Whitworth AJ, Pallanck LJ. The PINK1/ Parkinpathway: A mitochondrial quality control system? J Bioenerg Biomembr 2009;41:499– 503.
47. Zigmond M.J, Burke R.E, “Pathophysiology Of Parkinson’s Disease”, Neuropsycho pharmacology: The Fifth Generation of Progress.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









