 About Authors:
About Authors:
Satyanand Tyagi*
*President, Tyagi Pharmacy Association & Scientific Writer,
Chattarpur, New Delhi, India-110074.
Prof. Satyanand Tyagi is a life time member of various pharmacy professional bodies like IPA, APTI and IPGA. He has published various research papers and review articles. His academic works include 49 Publications (41 Review Articles and 08 Research Articles of Pharmaceutical, Medicinal and Clinical Importance, published in standard and reputed National and International Pharmacy journals; Out of 49 publications, 11 are International Publications).
*sntyagi9@yahoo.com, +91-9871111375 / 9582025220
ABSTRACT:
Erlotinib is a drug used to treat non-small cell lung cancer, pancreatic cancer and several other types of cancer. It is a reversible tyrosine kinase inhibitor, which acts on the epidermal growth factor receptor (EGFR). It is marketed in the United States by Genentech and OSI Pharmaceuticals and elsewhere by Roche. In lung cancer, it extends life by an average of 3.3 months at a cost of CDN $ 95,000. The hydrochloride salt of a quinazoline derivative of the drug shows antineoplastic properties. Competing with adenosine triphosphate, erlotinib reversibly binds to the intracellular catalytic domain of epidermal growth factor receptor (EGFR) tyrosine kinase, thereby reversibly inhibiting EGFR phosphorylation and blocking the signal transduction events and tumorigenic effects associated with EGFR activation. Erlotinib hydrochloride is approved to be used alone or with other drugs to treat:
* Non-small cell lung cancer that is locally advanced or has metastasized (spread to other parts of the body). It is used in patients who have already been treated with other chemotherapy.
* Pancreatic cancer. It is used with gemcitabine hydrochloride in patients whose disease cannot be removed by surgery or has metastasized.
Erlotinib hydrochloride is also being studied in the treatment of other types of cancer. The aim of present article is to provide in depth knowledge about the drug Erlotinib as well as its role in treatment of non-small cell lung cancer as well as pancreatic cancer.The review has also focused about chemistry, pharmacology as well as clinical trial studies of the Erlotinib.
[adsense:336x280:8701650588]
REFERENCE ID: PHARMATUTOR-ART-1469
INTRODUCTION:
Erlotinib hydrochloride (trade name Tarceva) is a drug used to treat non-small cell lung cancer, pancreatic cancer and several other types of cancer.
It is a reversible tyrosine kinase inhibitor, which acts on the epidermal growth factor receptor (EGFR). It is marketed in the United States by Genentech and OSI Pharmaceuticals and elsewhere by Roche. In lung cancer, it extends life by an average of 3.3 months at a cost of CDN$95,000. Chemically it is N-(3-ethynylphenyl)-6, 7-bis (2-methoxyethoxy) quinazolin-4-amine hydrochloride (Fig.1).
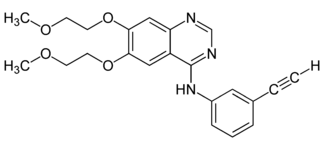
Fig.1:Chemical Structure of Erlotinib
[adsense:468x15:2204050025]
MECHANISM OF ACTION:
Erlotinib is an EGFR inhibitor. The drug follows Iressa (gefitinib), which was the first drug of this type. Erlotinib specifically targets the epidermal (EGFR) tyrosine kinase, which is highly expressed and occasionally mutated in various forms of cancer. It binds in a reversible fashion to the adenosine triphosphate (ATP) binding site of the receptor1. For the signal to be transmitted, two members of the EGFR family need to come together to form a homodimer. These then use the molecule of ATP to autophosphorylate each other, which causes a conformational change in their intracellular structure, exposing a further binding site for binding proteins that cause a signal cascade to the nucleus. By inhibiting the ATP, autophosphorylation is not possible and the signal is stopped.
CLINICAL APPLICATIONS:
Erlotinib has shown a survival benefit in the treatment of lung cancer in phase III trials. The SATURN (Sequential Tarceva in Unresectable NSCLC) study found that erlotinib added to chemotherapy improved overall survival by 19%, and improved progression-free survival (PFS) by 29%, when compared to chemotherapy alone2, 3. The manufacturer estimated that erlotinib can extend life by approximately 3.3 months. This is at a cost of CDN$95,000, which some researchers call "marginally”, cost effective4. In a draft guidance, NICE has recommended Tarceva for lung cancer5. The U.S. Food and Drug Administration (FDA) has approved for the treatment of locally advanced or metastatic non-small cell lung cancer that has failed at least one prior chemotherapy regimen. In November 2005, the FDA approved erlotinib in combination with gemcitabine for treatment of locally advanced, unresectable, or metastatic pancreatic cancer6.
In lung cancer, erlotinib has been shown to be effective in patients with or without EGFR mutations, but appears to be more effective in the group of patients with EGFR mutations.
A test for the EGFR mutation in cancer patients has been developed by Genzyme. The response rate among EGFR mutation positive patients is approximately 60%. Patients who are non-smokers, and light former smokers, with adenocarcinoma or subtypes like BAC are more likely to have EGFR mutations, but mutations can occur in all types of patients. EGFR positive patients are generally KRAS negative. Erlotinib has recently been shown to be a potent inhibitor of JAK2V617F activity. JAK2V617F is a mutant of tyrosine kinase JAK2, is found in most patients with polycythemia vera (PV) and a substantial proportion of patients with idiopathic myelofibrosis or essential thrombocythemia. The study suggests that erlotinib may be used for treatment of JAK2V617F-positive PV and other myeloproliferative disorders EGFR positive patients are generally KRAS negative. The drug's US patent will expire in 20208. In May 2012, the US District Court of Delaware passed an order in favour of OSI Pharmaceutical LLC against Mylan Pharmaceuticals upholding the validity of the patent for Erlotinib. In India, generic pharmaceutical firm Cipla is battling with Roche against the Indian patent for this drug.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
SIDE EFFECTS:
Common side effects
* Rash occurs in the majority of patients. This resembles acne and primarily involves the face and neck. It is self-limited and resolves in the majority of cases, even with continued use. Interestingly, some clinical studies have indicated a correlation between the severity of the skin reactions and increased survival though this has not been quantitatively assessed7. The Journal of Clinical Oncology reported in 2004 that "cutaneous [skin] rash seems to be a surrogate marker of clinical benefit, but this finding should be confirmed in ongoing and future studies8.
* “The newsletter Lung Cancer Frontiers reported in its October 2003 issue, "Patients with moderate to severe cutaneous reactions [rashes] have a far better survival, than those with only mild reactions and much better than those with no cutaneous manifestations of drug effects9.
* Diarrhoea
* Loss of appetite
* Fatigue
* Rarely, interstitial pneumonitis, which is characterized by cough and increased dyspnea. This may be severe and must be considered among those patients whose breathing acutely worsens.
* Rarely, ingrown hairs, such as eyelashes.
* It has also been suggested that erlotinib can cause hearing loss.
* Partial hair loss (by strands, not typically in clumps)
Rare side effects
In spring 2009, the US Food and Drug Administration issued a warning on erlotinib. The FDA reported serious gastrointestinal tract, skin, and ocular disorders in patients taking the drug. In addition, according to a letter released by Genentech and OSI Pharmaceuticals, some people prescribed erlotinib have developed serious or fatal gastrointestinal tract perforations; "bullous, blistering, and exfoliative skin conditions, some fatal; and serious eye problems such as corneal lesions. Some of the cases, including ones which resulted in death, were suggestive of Stevens–Johnson syndrome/toxic epidermal necrolysis.
DRUG INTERACTIONS:
Erlotinib is mainly metabolised by the liver enzyme CYP3A4. Compounds which induce this enzyme (i.e. stimulate its production), such as St John's wort, can lower erlotinib concentrations, while inhibitors can increase concentrations10.
RESISTANCE TO TREATMENT:
As with other ATP competitive small molecule tyrosine kinase inhibitors, such as imatinib (Gleevec) in CML, patients rapidly develop resistance. In the case of erlotinib this typically occurs 8–12 months from the start of treatment. Over 50% of resistance is caused by a mutation in the ATP binding pocket of the EGFR kinase domain involving substitution of a small polar threonine residue with a large nonpolar methionine residue (T790M)11. While proponents of the 'gatekeeper' mutation hypothesis suggest this mutation prevents the binding of erlotinib through steric hindrance, research suggests that T790M confers an increase in ATP binding affinity reducing the inhibitory effect of erlotinib12. Approximately 20% of drug resistance is caused by amplification of the hepatocyte growth factor receptor, which drives ERBB3dependent activation of PI3K. Other cases of resistance can involve numerous mutations, including recruitment of a mutated IGF-1 receptor to homodimerise with EGFR so forming a heterodimer.
This allows activation of the downstream effectors of EGFR even in the presence of an EGFR inhibitor. Some IGR-1R inhibitors are in various stages of development (based either around TKIs such as AG1024 or AG538 or pyrrolo[2,3-d]-pyrimidine derivatives such as NVP-AEW541). The monoclonal antibody figitumumab which targets the IGF-1R is currently undergoing clinical trials. The most promising approach to combating resistance is likely to be combination therapy. Commencing treatment with a number of different therapeutic agents with differing modes of action is thought to provide the best defence against development of T790M and other resistance conferring mutations.
FIRST LINE THERAPY IN NON-SMALL CELL LUNG CANCER:
The availability of targeted agents has become an invaluable resource in the treatment of advanced Non–small-cell lung cancer (NSCLC). The epidermal growth factor receptor (EGFR) plays an important role in the development and progression of NSCLC. In recent years the two small molecules, Erlotinib and Gefitinib, have been developed and extensively studied in patients with NSCLC. Both drugs are orally available small molecules that selectively and reversibly inhibit the tyrosine kinase domain of EGFR13. Although Erlotinib has modest clinical benefits after platinum-based chemotherapy in unselected patients with NSCLC, an emerging and potentially more elegant strategy is to move this agent to the frontline setting for selected patients. EGFR mutation status has emerged as the most important factor predictive of the benefit of first-line EGFR Tyrosine kinase inhibitor (TKI) therapy14. Erlotinib has been shown to improve progression-free survival compared with chemotherapy when given as first-line treatment for NSCLC patients with activating EGFR mutations15.
ERLOTINIB IN NSCLC: NCCN 2012 RECOMMENDATIONS:
As first-line therapy
* Indicated as a first-line therapy in patients with EGFR mutation
As maintenance therapy
* Initiation of Erlotinib after 4-6 cycles of first-line platinum-doublet chemotherapy
As second-line treatment
* In patients who have experienced disease progression either during or after first-line therapy, single-agent Erlotinib can be considered
As third-line treatment}
* Erlotinib is superior to best supportive care
PHASE-III CLINICAL TRIALS STUDY16
EGFR tyrosine kinase activating mutations are present in 10-26% of NSCLC tumours and are associated with increased response to gefitinib and erlotinib. However, little is known about how the efficacy and safety profile of erlotinib compares with CT in EGFR-mutant Caucasian p.
We have performed a prospective, randomized phase III study comparing erlotinib with platinum-based CT in chemonaive advanced NSCLC p with EGFR mutations.
Methods:
From February 2007 to January 2011, we screened 1,227 p for EGFR mutations, and 174 patients were randomly assigned to receive erlotinib or platinum-based CT.
The primary endpoint was progression-free survival (PFS). Secondary endpoints included response, overall survival and toxicity profiles.
Results: 153 p (76 CT, 77 erlotinib) are evaluable for the interim analysis. p characteristics CT arm: 16 males; median age, 64; never smokers, 56; PS 0, 26; PS 1, 41; adenocarcinoma, 67. p characteristics erlotinib arm: 25 males; median age, 65; never smokers, 54; PS 0, 23; PS 1, 44; adenocarcinoma, 73. Preliminary results of the interim analysis are now available. Response rate was 10.5% to CT vs 54.5% to erlotinib (P<0.0001). PFS in the CT arm was 5.2 months (m) (95%CI, 4.4-5.8 m) compared to 9.4 m (95%CI, 7.9-12.3) in the erlotinib arm (HR, 0.42; P<0.0001). Median survival was 18.8 m in the CT arm and 22.9 m in the erlotinib arm (HR, 0.80; P=0.42). Most common toxicities were asthenia (68.9%), anaemia (45.9%), nausea (40.5%) and neutropenia (36.5%) in the CT arm, and diarrhoea (57.3%), asthenia (53.3%), and rash (49.3%) in the erlotinib arm. Final results of the interim analysis will be presented.
Conclusions: The EURTAC study met its primary endpoint at the interim analysis. Erlotinib as first-line treatment for advanced NSCLC p with EGFR mutations improves PFS, with acceptable toxicity, compared to platinum-based chemotherapy.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
SUMMARY AND CONCLUSION:
Advances in understanding the tumour biology have led to the use of EGFR TK inhibitors in patients with advanced NSCLC. Studies with these agents defined NSCLC patient subsets particularly likely to respond to these agents, including women, non-smokers, Asians, and those with adenocarcinoma histology. These characteristics are a substitute for a higher prevalence of EGFR-mutated tumours, but the decision to use an EGFR TK inhibitor as first-line therapy should be made based upon molecular testing rather than clinical criteria whenever possible. For patients with previously untreated metastatic NSCLC and an activating mutation of the EGFR, monotherapy with an EGFR TK inhibitor Erlotinib is recommended rather than chemotherapy. In randomized trials, this approach significantly prolongs progression-free survival. In the OPTIMAL trial, 154 patients with known EGFR mutations and measurable disease, treatment with Erlotinib significantly improved progression-free survival than treatment with Gemcitabine plus Carboplatin (13.1 vs. 4.6 months). Similarly, the objective response rate was significantly improved with Erlotinib (83 vs. 36%).
The results of Erlotinib expanded access program and similar trials, collectively referred to as the TRUST study, using Erlotinib treated in a routine clinical setting among large unselected population with advanced NSCLC, confirm the activity and favourable tolerability of Erlotinib. Taken as a whole, Erlotinib is very effective and well tolerated in advanced NSCLC patients who harbor EGFR activating mutations. It is 2 to 3 times more effective than doublet chemotherapy. For patients with advanced lung cancer whose tumours carry EGFR activating mutations, first-line treatment with Erlotinib nearly tripled progression-free survival compared to a standard chemotherapy combination.
REFERENCES:
1. Raymond E, Faivre S, Armand J (2000). "Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy". Drugs, 60 Suppl 1: 15–23; discussion 41–2.
2. 2009 - SATURN: A double-blind, randomized, phase III study of maintenance erlotinib versus placebo following nonprogression with first-line platinum-based chemotherapy in patients with advanced NSCLC.
3. April 2010 - Tarceva Indication Announcement Letter
4. Erlotinib for Advanced NSCLC is "Marginally" Cost Effective, Fran Lowry, Medscape, 25 February 2010.
5. Takimoto CH, Calvo E. "Principles of Oncologic Pharmacotherapy" in Pazdur R, Wagman LD, Camphausen KA, Hoskins WJ (Eds) Cancer Management: A Multidisciplinary Approach. 11 ed. 2008.
6. Li Z, Xu M, Xing S, Ho W, Ishii T, Li Q, Fu X, Zhao Z (2007). "Erlotinib Effectively Inhibits JAK2V617F Activity and Polycythemia Vera Cell Growth". J Biol Chem, 282 (6): 3428–32.
7. Dudek A, Kmak K, Koopmeiners J, Keshtgarpour M (2006). "Skin rash and bronchoalveolar histology correlates with clinical benefit in patients treated with gefitinib as a therapy for previously treated advanced or metastatic non-small cell lung cancer". Lung Cancer, 51 (1): 89–96.
8. Román Pérez-Soler, M.D., et al. (2004). "Selected Highlights". Lung Cancer Frontiers, 22 (16): 3238–3247.
9. Thomas L. Petty, M.D. (2003). "Determinants of Tumour Response and Survival with Erlotinib in Patients with Non—Small-Cell Lung Cancer". Journal of Clinical Oncology, 1 (17): 3–4.
10. Haberfeld, H, ed. (2010) (in German). Austria-Codex (2010/2011 ed.). Vienna: Österreichischer Apothekerverlag.
11. Balak MN, Gong Y, Riely GJ, Somwar R, Li AR, Zakowski MF, Chiang A, Yang G, Ouerfelli O, Kris MG, Ladanyi M, Miller VA, Pao W (2006). "Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors.". Clin Cancer Res, 12 (1): 6494–501.
12. Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, Meyerson M, Eck MJ (2008). "The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP.” PNAS, 105 (6): 2070–5.
13. Available at medscape.com/viewarticle/755979
14. Clin Lung Cancer, 2012; 13(2):107-14.
15. Lancet Oncol, 2012; 13(3):239-46.
16. J Clin Oncol, 29: 2011
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









