ABOUT AUTHORS
Pomila*; Anjali Sidhu
Department of chemistry,
Punjab Agriculture University,
Ludhiana, Punjab, India
ABSTRACT
The current review frameworks different approaches of synthesis and various biological activities of allyl sulfides. The food-based natural products (allyl sulfides) are major organo-sulfur constituent of garlic had been studied extensively due to their moderate toxicity accompanied by number of biological applications such as anti-cancer, anti-microbial, antibiotic, antimutagenic and detoxification etc. The broad-spectrum application of allyl sulfides inspires us to do advance research on it.
Reference Id: PHARMATUTOR-ART-2678
INTROUDCTION:
Garlic (Allium sativum L.) is widely used in traditional herbal remedies and alternative medicine for centuries (Rivlin 2001). The potential health benefits of garlic such as antibacterial, antimicrobial, antifungal, antioxidant and anti-tumour are largely attributed to its organo-sulfur compounds. Among organosulfur compounds allyl sulfides such as diallyl monosulfide, diallyl disulfide, diallyl trisulfide, diallyl tetrasulfide, diallyl penta and hexasulfides, methyl allyl sulfide, ajoene ,vinyldithins etc are major organo-sulfur compound found in garlic known for their unique therapeutic properties such as anticancer (due to their lipid soluble nature), antimutagenic, detoxification (Seki et al., 2012), antibiotic and antibacterial (Casella et al., 2013) metal binding and catalytic activities. These are considered to be most active moieties along with key advantage of moderate toxicity (Anwar et al., 2017). Sulfur present in allyl sulfide is termed as sulfane sulfur attributed to the extraordinary biological potential of allyl sulfides.(Toohey J.I 1989). The S-S bond linkage found in allyl sulfides open a new gate way as potent anticancer agents in modern medicinal chemistry (Allah et al., 2015, Dvorkova et al., 2015, Dong et al., 2014 and Nakagawa et al., 2001). The reason behind the remarkable biological applications of allyl sulfides are their sensitivity towards the cellular components of living system (Venkatesh et al., 2018 ). A series of independent studies indicates that in comparison to normal cells, cancer cells show more sensitivity towards the treatment of diallyl monosulfide and diallyl disulfide (Nakagawa et al., 2001)
Review
A. SYNTHESIS OF ALLYL SULFIDES
Bhaumik et al., 2017 synthesized symmetrical disulfides involving rapid conversion of alkyl halides into disulfides (1) by reaction of sodium sulfide and carbon disulfide in DMF at room temperature.
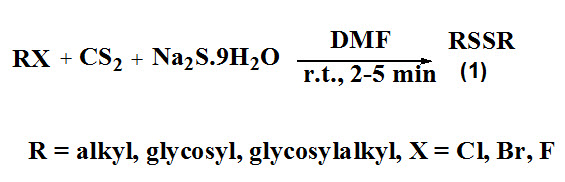
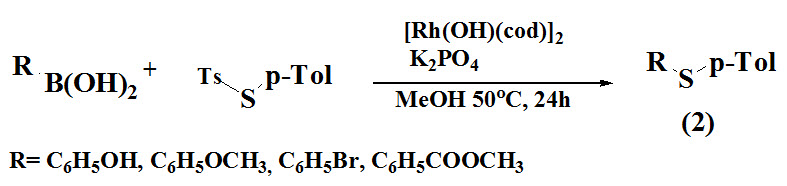
Kanemoto et al., 2017 reported odourless procedure for diaryl sulfides (2) using treatment of S-aryl thiosulfonates with borylarenes followed by deborylthiolation using rhodium as a catalyst.
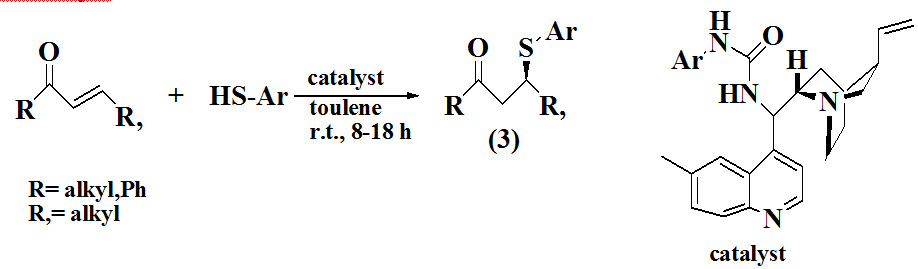
Grayson et al., 2016 reported synthesis of optically active sulfides (3) by carried out conjugate addition between thiols and various α,β-unsaturated in the presence of urea organocatalyst derived from cinchona alkaloid.
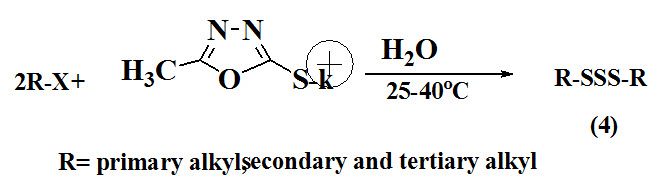
Mohammadi and Beigi 2016 proposed simple and green method for synthesis of symmetrical trisulfides (4) from alkyl halides in water and using PMoxt as a sulfur donor.
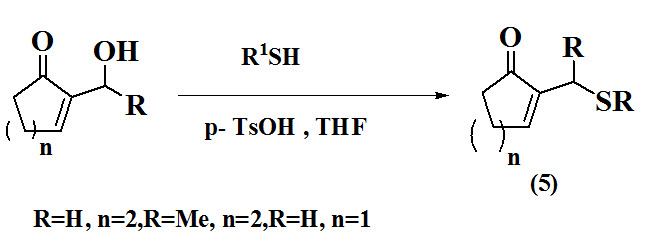
Oueslati et al., 2016 reported a direct route for synthesis of allyl sulfides (5) using cyclic p-TsOH-mediated followed by the direct α-substitution of cyclic Morita–Baylis–Hillman alcohols with aliphatic and aromatic thiols in refluxing THF. The reaction proceeded with complete α-regioselectivity and provided the corresponding allyl sulfides in moderate to good yields.
Selvaraj et al., 2016 synthesized diallyl sulfide (6) using ultrasound assisted multisite phase transfer catalyst 1,3,5,7-tetrabenzylhexamethylenetetraammonium tetra bromide (MPTC) which was prepared by carrying out reaction between hexaethylenetetramine and benzyl bromide in presence of ethanol.

Wu et al., 2016 reported an efficient pathway for synthesis of aryl methyl sulfides (7) via coupling of arylboronic acids with dimethyl disulfide under metal-free conditions.
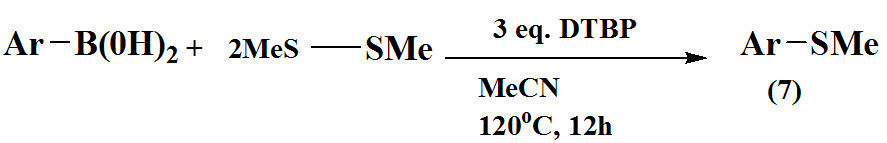
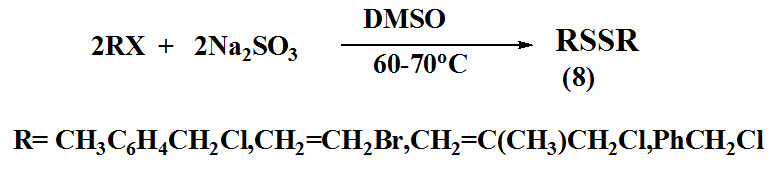
Abbasi et al., 2016 reported synthesis of symmetrical disulfides (8) by reacting organic halides and Na2SO3 in DMSO at 60-70ºC. The progress of the reaction checked by litmus paper, the color of litmus paper changed from yellow to red which indicated formation of product.
Gosh et al., 2015 reported a convenient method for the synthesis of aryl methyl sulfides (9) via cu (I)-mediated methylthiolation of haloarenes with DMSO which act source of sulphur.
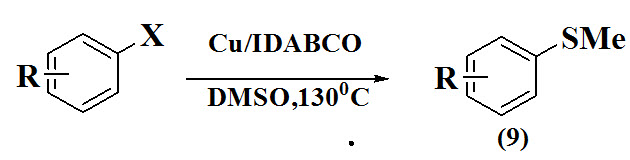
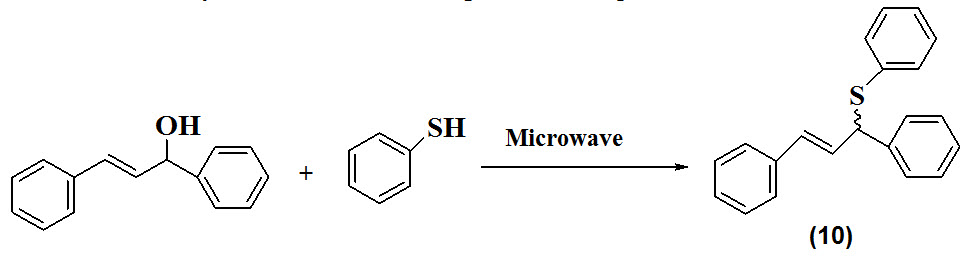
A new solvent and catalyst free approach of synthesis of disulfides (10) reported by Tabarelli et al., 2014. Prior to the synthesis 1,3-diphenylprop-2-en-1-ol and benzenethiol were made to react in the absence of solvent and catalyst followed by microwave irradiations for 20min. The reaction was very sensitive towards temperature change.
Baker et al., 2013 prepared symmetrical disulfides (11) using phase transfer catalysis. Disodium disulfide prepared in situ by carrying reaction between sodium sulfide and sulphur at 50°C in water, with addition of TBAB (Tetrabromide ammonium bromide) as phase transfer catalyst in ethanol. Trisulfides and other polysulfides were also synthesized by using same scheme by varying the concentration of sulfur.

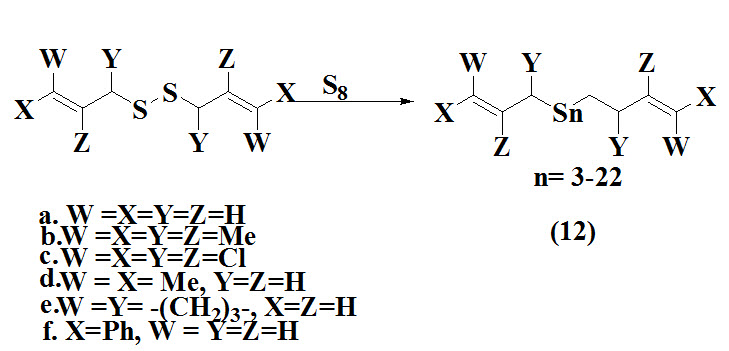
Wang et al., 2013 synthesized diallyl polysulfanes (12) by treatment of diallyl disulfide and liquid sulphur at 120ºC resulted into families of polysulfanes with up to 22 sequential sulphur atoms. The resulted product characterized by ultra-performance liquid chromatography and obtained in good yield.
Nishimoto et al., 2012 reported an efficient method for synthesis of allyl sulfides (13) via substitution of the acetoxy group in alkyl, benzyl, allyl, and propargyl acetates with thiosilanes in the presence of indium triiodide as catalyst. The product was obtained in good yield.
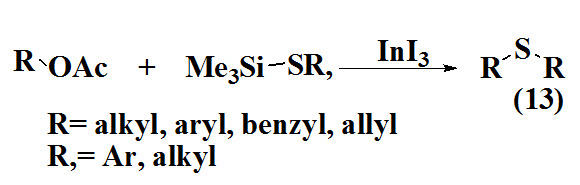
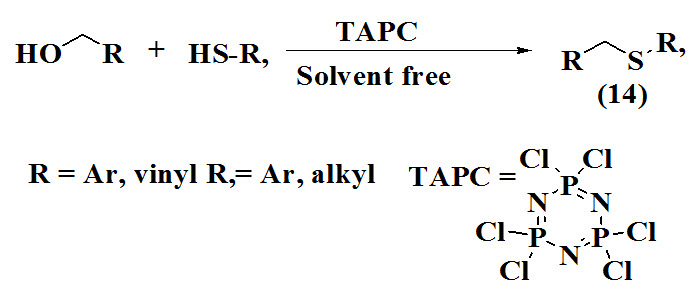
Bahrami et al., 2011 proposed efficient synthesis of allyl sulfides (14) using TAPC as catalyst by reaction of benzylic alcohols with aryl, heteroaryl, and alkyl thiols under metal-free and solvent-free conditions. The products were obtained in good yield and were characterized by spectrometric techniques.
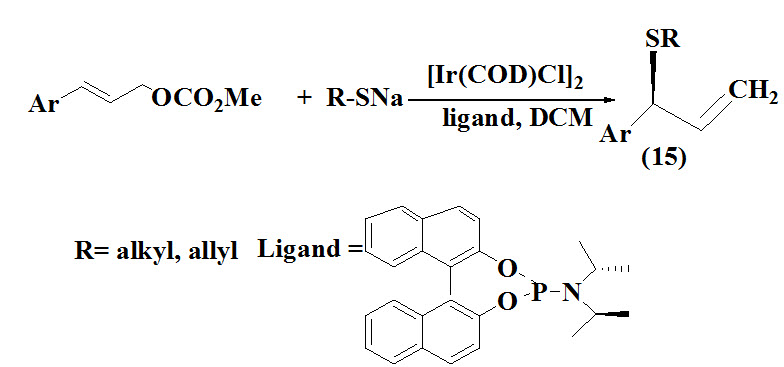
Gao et al., 2011 reported an iridium-catalyzed regio-and enatioselective synthesis of allyl sulfides (15) via allylation of allyl carbonates with aliphatic thiols as the nucleophile in dichloromethane enables the regioselective synthesis of branched allyl sulfides in good yields and high enantioselectivity.
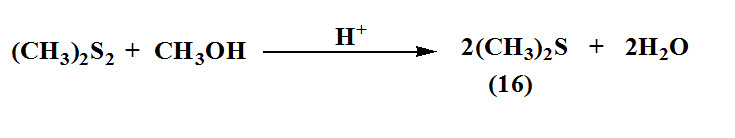
Mashika, 2011 reported new method for synthesis dimethyl sulfide (16) by treatment of dimethyl disulfide with methanol in the presence of solid catalyst, aluminum γ-oxide. The yield of dimethyl sulfide depend upon temperature, contact time, and content of methanol in the reaction mixture. The product obtained in good yield at room temperature and characterized by mass spectrometry and FT-IR.
Lee et al., 2011 reported cross-coupling reactions between carbon and sulfur with indium tri(organothiolates) to synthesis di-, tri- and tetrasulfides (17) in a one-pot procedure which involve reaction of indium tri(organothiolates) with polybromonated aromatic and hetero aromatic compounds.
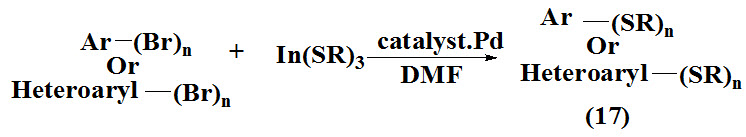
Qiao et al., 2011 proposed efficient synthesis of aryl methyl sulfide (18) by carried out methylthiolation using (methylthio)trimethylsilan. To a solution of 1-iodo-3,5-dinitrobenzene in DMSO was added Cs2CO3 and followed by stirring for 2 min. A dark purple solution obtained and TMSSMe was added.
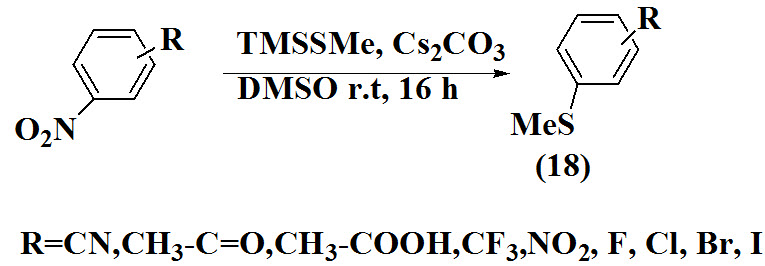
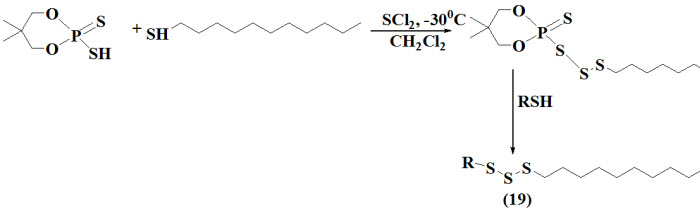
et al., 2010 reported synthesis of unsymmetrical trisulfides (19) followed by treatment of SCl2 with mixture of dodecane-1-thiol and 5,5-dimethyl-2-sulfanyl-2-thioxo-1,3,2-dioxaphosphorinane at 30°C and resulting intermediate 1-[(5,5-dimethyl-2-thioxo-1,3,2-dioxaphosphorinan-2-yl)-trisulfanyl]dodecane11-sulfanylundecanol. The intermediate further afforded a complex mixture of product such as unsymmetrical and symmetrical trisulfides on treatment with Et3N.
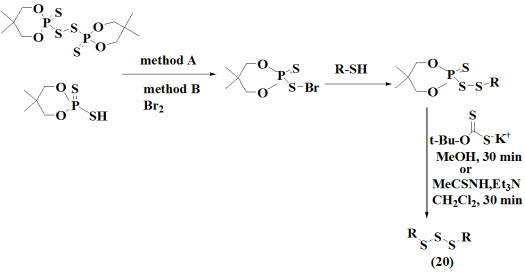
Kertmen et al., 2009 proposed a novel and efficient method for synthesis of symmetrical trisulfides (20) with treatment of bis (5,5-dimethyl 2-thioxo-1,3,2-dioxaphosphorinan-2-yl) disulfide or 5,5-dimethyl-2-sulfanyl-2-thioxo-1,3,2-dioxaphosphorinane with bromine at 30 °C resulting into desirable products.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
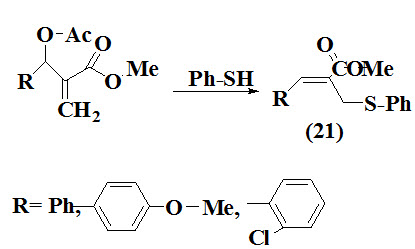
Das et al., 2007 reported synthesis of (Z)- and (E)-allyl sulfides (21) using Morita-Baylis-Hillman Acetates. The product obtained in good yield and characterized by various spectrometric techniques. The reaction occurs at room temperature and without presence of any catalyst.
Yatusmonji et al., 2007 synthesized allylic sulfides (22) by treatment of allylic acetates with thiols in the presence of nickel (0) triethyl phosphite complex with retention of configuration without allylic rearrangement.

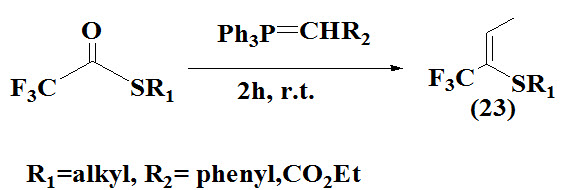
Begue et al., 1996 prepared Trifluromethyl vinyl sulfides (23) by witing reaction on thiotrifluoroacetates.
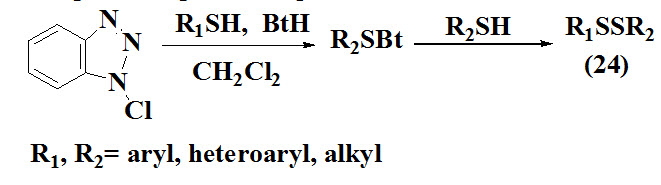
The one step formation of unsymmetrical disulfides (24) reported by Hunter et al., 2006 which involved oxidation of 1-chlorobenzotriazole (BtCl) in the presence of CH2Cl2 at -78ºC and different thiols agents. The products were obtained in moderate yield. The product was analysed by various spectroscopic techniques.
Zhan and Lang, 2004 proposed a new convenient synthetic procedure for allyl sulfides (25) which involve treatment of alkyl thiocyantes or aryl disulfides with allyl bromide in the presence of samarium iodide as a single electron transfer reagent as shown above:

Ren et al., 2009 reported synthesis of 1,3-Diallyltrisulfane (26) by carried out reaction between sodium thiosulfate and unsaturated alkyl bromide at 500-600C with continuous stirring followed by addition of sodium sulfide solution. The product obtained in good yield.
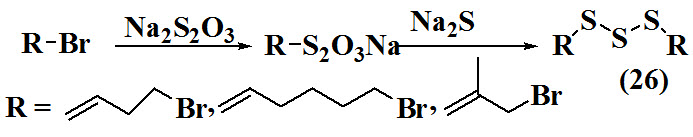
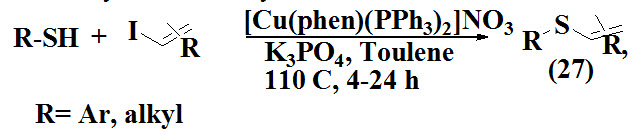
Bates et al., 2004 proposed a palladium-free approach for the synthesis of vinyl sulfides (27) in presence of copper(i) catalyst [cu(phen)(pph3)2]NO3, the desired product are observed with retention of stereochemistry in excellent yield.
Sinha et al., 2003 synthesized diorganotrisulfides (28) by carried out reaction of organic halide and elemental sulfur using catalytic copper (II) halide and Tin (II) chloride in the solution of DMSO and THF at 30-70ºC.
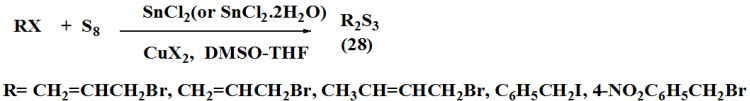
Prakash et al., 2002 reported synthesis of aryl phenyl sulfides (29) and aryl methyl from arenediazonium tetrafluoroborates and trimethyl(methylthio)-and trimethyl(phenylthio)silanes. A solution of trimethyl(phenylthio)-silane in dimethylformamide was added drop wise over 20 min to a solution of the corresponding diazonium tetrafluoroborate salt in DMF at –10°C (ice/salt mixture bath).
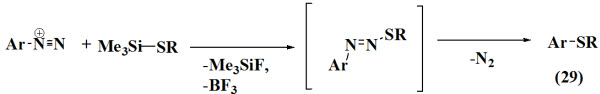
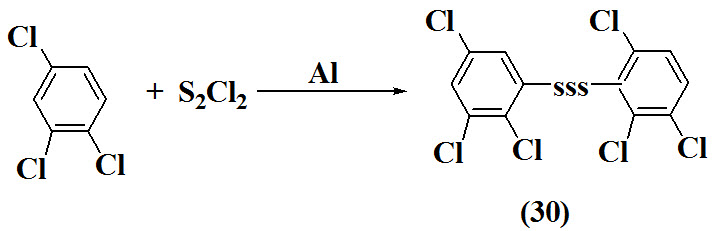
Ayodele et al., 1999 synthesized aryl trisulfides (30) by treatment of 1,2,4-trichlorobenzene with sulfur monochloride and aluminurn gives the S,S'-trisulfide..
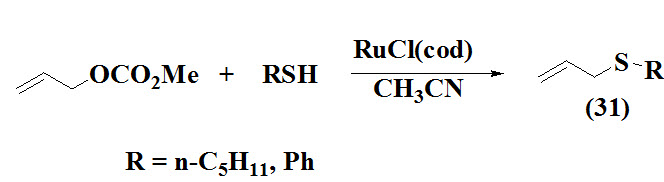
Kondo et al., 1999 reported first ruthenium –catalyzed synthesis of allylic sulfides (31) via allylation of thiols involving treatment of aliphatic/ aromatic thiols with allyl methyl carbonate in the presence of RuCl(cod) in CH3CN at room temperature under argon atmosphere.
Ogawa et al., 1999 studied stereocontrolled and region formation of vinyl sulfides (32) via reaction of thiols with alkynes using transition-metal-Catalysis at 25°C. After the reaction was complete the solvent was removed in vacuo. Purification was done by a recycling preparative HPLC.
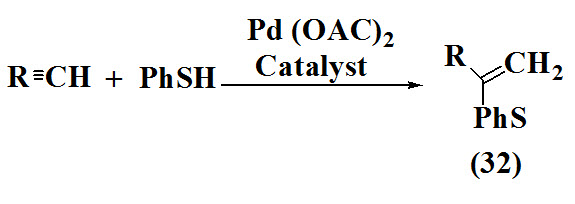
Zhan et al., 1999 reported a novel synthesis of allyl sulfides (33) via. Sm-BiCl3 system in aqueous media. The best yield were obtained under nitrogen atmosphere. The result indicated that Sm-BiCl3 system facilitates the cleavage of S-S bond which was considered one of the major advantage of this method. The products were characterized by various spectroscopic techniques.
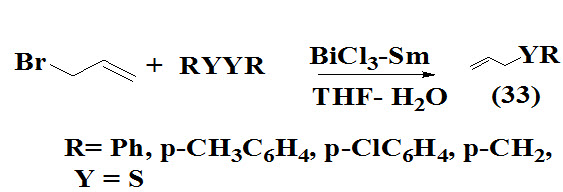
Mingxin and Zhang, 1997 reported a convenient procedure for allyl sulfides (34) which involve treatment of alkyl or aryl disulfides in the presence of samarium as a catalyst with allyl bromide in THF lead to product in good yield.
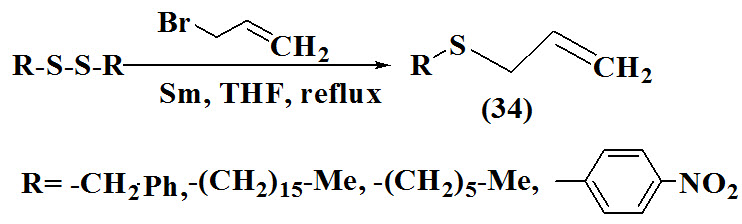
A new synthetic route for synthesis of disulfides (35) reported by Wu et al., 1996. The reaction was carried out between thiols and bromine which act as a oxidizing agent without use of any solvent. The reaction conditions were mild and the yields were essentially quantitative.
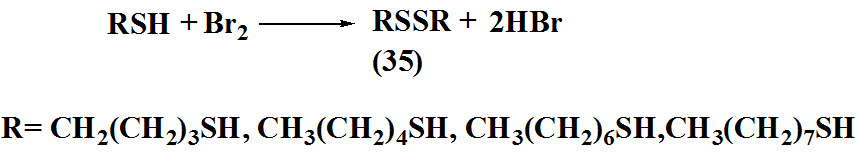
Archer et al., 1994 reported synthesis of sulfide (36) from camphor by treatment of camphor with a thiol agent in the presence of a lewis acid (F3B.OH2) result into formation of thionium ion as a intermediate further reacted with Et3SiH to form chiral sulfides.
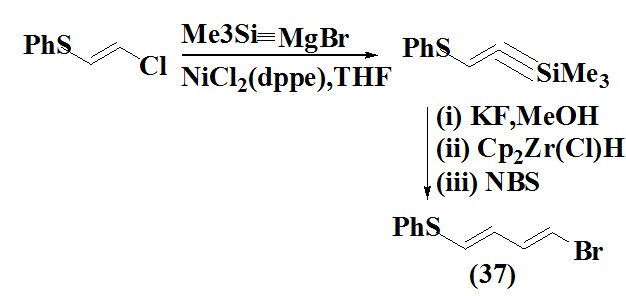
Babudri et al., 1998 synthesized vinyl aryl sulfides (37) using conjugated (E,E)-1-phenylthiobuta-1,3-dienes as a intermediate formed by treatment of trimethylsilylalkyne with Ni11- based cross coupling between Grignard reagents and 1-chloro-2-phenylthioethene. The intermediate undergo desilylation and treated with NBS (N-Bromosucinide) to give product.
Backvall et al., 1994 synthesized vinyl sulfides (38) via palladium catalysed thioboration of alkynes with 9-(alkylthio)-BBN. The products were obtained in good yield
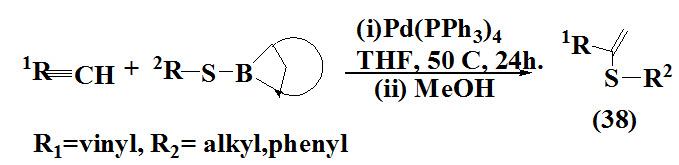
Goux et al., 1994 reported synthesis of allyl, homoallyl sulfides (39) by approaching various methods. The allylation reaction of thiols using carbonated and aromatic thiols in the presence of Pd0 gives moderate to excellent yield of allyl and cinnamyl aryl sulfides.
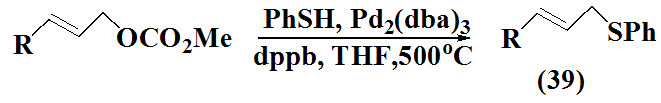
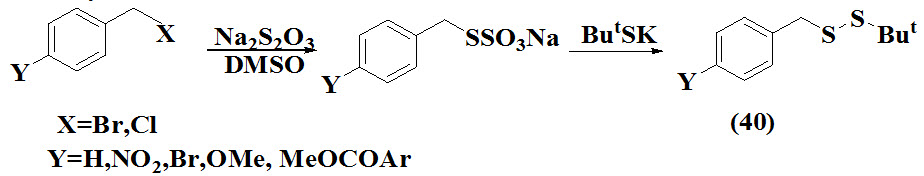
Hiver et al., 1994 proposed synthesis of unsymmetrical benzylic disulfides (40) from benzylic bromides and chlorides derived bunate salts. The reaction involve chemical treatment of thiosulfinate in DMSO subsequently followed by addition of thiolate to generate unsymmetrical disulfide.
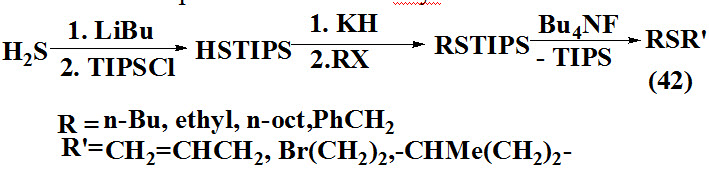
Miranda et al., 1994 reported synthesis of unsymmetrical sulfides (42) using a new reagent trisopropylsilanethiol (TIPS) which formed by conversion of H2S to triisopropylsilyl (HSTIPS) further treated with potassium salt and akyl halide
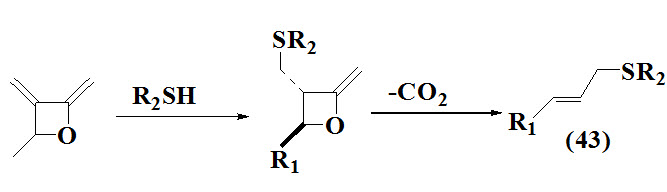
Another interesting method allyl sulfides (43) reported by Nava-Salgado et al; 1995 involve extrusion of CO2 from the intermediate (substituted ß- lactone) with retained stereochemistry as reactant resulted into main product.
Richter et al., 1994 reported synthesis of disulfides (44) by carried out benzylation of different thiols sources.

Coldham et al., 1993 reported formation of allylic sulfides (45) via formation of a intermediate (episulfonium ion) by shift of a phenylthio group from ß-hydroxy sulfide followed by removal of a proton and resulted into achievable product.

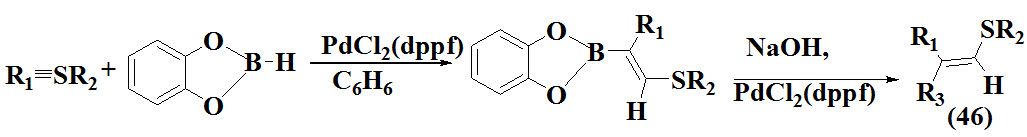
Gridnev et al., 1993 reported one pot method for synthesis of vinyl sulfides (46) using thioalkynes via cataltytic hydroboration.The synthetic procedure involve reaction of a thioalkyne in presence of Pd0 with catechol borane resulting into main product.
Meshram, 1993 reported synthesis of disulfides (47) through cleavage of thiol acetate by clayfen (Fe (NO)3) in the absence of solvent at room temperature. The product was extracted with dichloromethane and purified with column chromatography. The non-solvent feature of this method promoted solid phase synthesis.

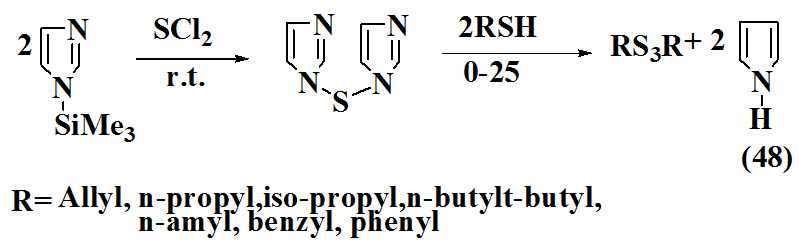
Banerji and Kalena, 1980 synthesized organic trisulfides (48) with addition of hexane solution of n-trimethylailylimidazole with sulfur dichloride in hexane under dry conditions followed by addition of freshly distilled allyl thiol.
Meijer and Vermer, 1974 synthesized substituted disulfide ie. (1-(methy1thio)propyl propenyl disulfide ) (51) at -300C by adding butyllithium with mixture of of cis/trans-l-propenethio in dry ether followed by continuous stirring of ten minutes. The desirable product was obtained on pouring the reaction mixture on ice and possessed almost same configuration as same asreactant.

Oswald et al., 1968 synthesized allyl sulfides (52) via addition of dithiols to allene involved free radical mechanism lead to polymer formation. The polyadditions were usually followed by nuclear magnetic resonance (NMR) spectroscopy of samples from the reaction mixtures. The crude liquid products were stripped with nitrogen bubbling at 1350C. under a pressure of about 0.3 mm. to remove all the volatile starting materials and impurities. The solid polyadducts were purified by fractional reprecipitation with a benzene-methanol solvent-precipitant pair.
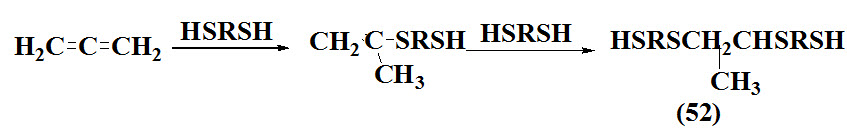
Moore et al., 1963 prepared allyl sulfides by carried out reduction of cis-1,3-dimethylbut-2-enyl disulfide (53) and trans-2-methylpent-2-enyl disulfide (54) in the presence of triphenylphosphine (PPh3) at 80ºC for 96 hr. The organic sulfides were isolated by chromatography.

The organic disulfides (55) can be prepared by thioalkylation of thiosulfinates in the presence of thio agents Nakabayashi 1961. The reaction of sulfenic sulfonic thioanhydride with p-toulenethiol produced dipropyltrisulfide at room temperature. The product was purified with column chromatography and obtained as a pale yellow color oil.

NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
C. Biological Properties
Extensive research has shown that oil soluble allyl sulfides present in garlic responsible for high biopotential (Iciek et al., 2012). Diallyltrisulfide and other allyl sulfides such as diallyl disulfide, diallyl pentasulfide and diallyl hexasulfidedomestically marketed in china since 1981 for treatment of fungal, parasitic and bacterial infections in man especially organo-sulfur compounds derived from garlic exhibit anticancer, antibacterial, antimicrobial,antivirus and antioxidant properties, immune system stimulation, cholesterol reducing properties (Johnson et al., 2016, Mnayer et al., 2014, Wang 2012)
Antimicrobial activity:
Li et al., 2015 studied antimicrobial activity of fresh garlic extract against Pseudomonas aeruginosa and Candida albicans. The result showed that Fresh garlic extract displayed evident inhibition properties against C. albicans yet weak inhibition properties against P. aeruginosa.
Casella et al., (2013) studied the role of diallyl and dipropyl sulfides in vitro antimicrobial activity against P. aeruginosa and E. coli. The antimicrobial activity was evaluated by measuring growth of inhibition zone. The result indicated that presence of CH2=CHCH2 group in sulfides structure contributed to its antimicrobial activity as dipropyl sulfides did not show any antimicrobial activity due to complete absence of CH2=CHCH2 group. The antimicrobial potent of the DPDS (dipropyl disulfide) and DADS (diallyl disulfide) were also studied. The results showed presence of the allyl moiety responsible for antimicrobial effect of sulfide derivatives . The DADS showed inhibition zone of 15.9mm on S. aureus and for P. aeruginosa was 21.9 mm where as for E. coli inhibition zone was 11.4 mm.
Alli et al., 2011 studied mode of action of crude garlic (Allium sativum) and its antimicrobial effects on clinical isolates of S. aureus and P. aeruginosa and further compared with strains of Staphylococcus aureus ATTC 25923 as control. The minimum bactericidal concentration (MBC) and minimum inhibitory concentration (MIC) were measured using agar dilution method for isolates of S. aureus and P. aeruginosa. The experimental investigation showed zone of inhibition was 201mg/ml for S. aureus followed by 134mg/ml for P. aeruginosa.
Rattanachaikunsopon et al., 2009 studied antimicrobial activity of elephant garlic oil against 15 different strains of V.cholera. The estimated sulfide content of the oil (diallyl monosulfide- 1.62%, diallyl disulfide-25.09%, diallyl trisulfide- 16.04%, diallyl tetrasulfide- 10.58%) and their activity compared with commercially available diallyl tetrasulfide, diallyl trisulfide, diallyl disulfide and diallyl monosulfide. The result showed that obtained MIC value varied according to the different strains of V.cholera ranging from 25 to100 µg/ml for diallyl for diallyl disulfide, 1.57 to 12.5 µg/ml for diallyl disulfide, 0.2 to 1.57 µg/ml for diallyl trisulfide and 0.005 to 0.4 µg/ml for diallyl tetrasulfide.
The antimicrobial activity of diallyl sulfide content in chive oil studied by Rattanachaikunsopon et al., 2008 against food-borne pathogenic bacteria Bacillius cereus, Clostridium botulinum, Listeria monocytogenes, Salmonella enterica, S.aureus and Vibrio cholera. The results indicate that diallyl sulfides considered to be responsible for antimicrobial activity in Alliaceae family, but due to their low content in chive oil diallyl sulfide exhibited low antimicrobial activity.
Leuschner et al., 2003 reported antimicrobial effects of garlic oil, clove and red hot chilli on Listeria monocytogenes (food borne pathogen). The garlic and clove both exhibited bacteriocidal activity where as red chilli found to be less inhibitory. The result indicate that garlic kill 50% colonies of L. monocytogenes at concentration of 3×106 CFU/ml where as clove show activity at concentration of 1×103 CFU/ml.
Ross et al., 2001 examined antimicrobial effect of garlic oil and powder. The result indicated that antimicrobial may involve sulfhydryl reactivity. All bacteria tested, which included both gram-negative and -positive bacteria and pathogenic forms, were susceptible to garlic materials. On the basis of weight-of-product basis, 24 h MICs for GO (0.02 to 5.5 mg/ml, 62 enteric isolates) and dimethyl trisulfide (0.02 to 0.31 mg/ml, 6 enteric isolates) were lower than those for a mixture of diallyl sulfides (0.63 to 25 mg/ml, 6 enteric isolates) and for GP, which also exhibited a smaller MIC range (6.25 to 12.5 mg/ml, 29 enteric isolates).
Tsao et al., 2001 studied In-vitro antimicrobial activity of four diallyl sulfides diallyl monosulfide, diallyl disulfide, diallyl trisulfide, diallyl tetrasulfide respectively against Aspergillus spp., Staphylococcus aureus, methicillin-resistance S.aureus, and Candida spp. The magnitude of activity of four diallyl sulfides followed the order diallyl monosulfide < diallyl disulfide < diallyl trisulfide < diallyl tetrasulfide showed that their antimicrobial activity directly related to no. of disulfides bonds, the more disulfide bonds greater the antimicrobial activity.
Avato et al., 2000 studied allylsulfides components of garlic oil as antimicrobial agents against Candida spp, Blastoschizomyces capitatus, Bacillus subtilis, S. aureus, E. Coli and P.aeruginosa. The result showed that out of all the component of garlic oil diallyl trisulfide and diallyl disulfide found to be more potent antimicrobial agent with different MIC values . The garlic extract containing DDS (41%) and DTS (40%) exhibited antimicrobial action against C. spp with MIC value 0.8µg/ml followed by MIC value 0.25µg/ml against B.subtilis, S. aureus and P.aeruginosa. It also concluded that none of the garlic extract found to active aginst growth of E. Coli.
Antibacterial activity:
Soltan et al., 2016 examined antbacterial activity of garlic essential oil against four pathogenic bacteria Agrobacterium tumefaciens, Erwinia amylovora, Rhodococcus fascians and Corynebacterium fascians by Agar dilution method. The result indicated that garlic oil crudes (diallyl monosulfide, diallyl disulfide, diallyl trisulfide, diallyl tetrasulfide) exhibited stongest pramylovorawith MIC value 300 µg/ml.
Liu et al., 2010 studied antibacterial mode of action of allitridin (diallyl trisulfide) against Heliocobacter pylori infection. The studies indicated allitridin exhibited inhibitory effect on H.pylori in concentration-dependent manner. In 24 hours time course examination 1µg/ml dose of allitridin was enough for its inhibitory action. The further study on the inhibitory action of allitridin indicate that the two proteins of H.pylori namely cytotoxin-associated gene A and neutrophil-activating protein were down regualted by allitridin which was reason for its inhibitory action.
Gara et al., 2000 reported activities of garlic powder (GP), garlic oil (GO) and their diallyl constituents against different strains of H.pylori. The garlic material exhibited anti H. pylori effects with variable MICs value (8 to 32µg/ml) as well as MBCs value (16 to 32µg/ml).The MICs and MIBs values of GO found to be lower than MICs and MIBs value of GP due to presence of allicin. Allicin with (MIC 6µg/ml; MIB 6µg/ml) values was more potent than diallyl disulfide content of GO with (MIC 100µg/ml; MIB 100µg/ml), However diallyl tetrasulfide show similar result as allicin with (MIC 3-6µg/ml; MIB 3-6µg/ml) values and indicated that antimicrobial effect of diallyl sulfides increases with increase in the no. of sulfur atoms.
Jonker et al., 1999 studied antibacterial impact of two different garlic extracts, home-made raw garlic extract and commercially available garlic tablets alone and in accordance with omeprazole or antibiotics contrast to clinical isolates of H pylori. The study indicated that MIC values of the commercial tablets were based on the allicin content, no differences between the three types were observed. The amalgam of omeprazole and showed a synergistic effect which was concentration dependent. The MIC values ranged from 10,000 to 17,500 mg/L
Nematicidal activity:
Anastasiadis et al., 2011 studied effect of garlic oil component (diallyl disulfide) and entomopathogenic nematodes as biocontrol agent on root- knot nematodes on tomato (Meloidogyne javanica). The result indicate that diallyl disulfide showed more effective
nematicidal activity.
Park et al., 2007 reported nematicidal activity of components from garlic and plant essential oils and cinnamon oil against the pine wood nematode (Bursaphelenchus xylophilus). The nematicidal activity varied according to compound dose, stage and sex of nematode. The compound found to be most toxic to pine wood nematode was diallyl trisulfide with LC50 value 3.72, 2.79 and 2.79 µlL-1 followed by cinnamyl acetate (from cinnamon oil) with LC50 value 39.30 µlL-1, diallyl disulfide with LC50 value 43.20, 46.48, 37.06 µlL-1 against male, female and juvenile nematode respectively.
Antifungal activity:
The antifungal evalution of garlic oil was checked against Candida albicans by multiple methods including the poisoned food technique. The garlic oil due to its antifungal oragnosulfur components (diallyl monosulfide, diallyl trisulfide, diallyl disulfide and diallyl tetrasulfide) showed effective antifungal activity with MIC value 0.35µg/ml. The Transmission electron microscopy revealed penetration of cellular membrane of C. albicans as well as in membranes of organelles like mitochondria, resulting into ultimate cell death. The test fungi further analysed for its RNA sequence which showed that garlic oil caused the destruction of those critical genes which involved in oxidation-reduction processes (Li et al., 2015).
The antifungal effect of garlic oil on Penicillium funiculosum examined by Li et al., 2014 using poisoned food technique. The GC/MS analysis of garlic oil indicate that sulfides, disulfides, trisulfides were the main consituents of the oil which hold for its biological activity. The result showed that 0.0313% (v/v) garlic oil can kill 5×105 CFU/ml of P. funiculosum conidia within 6 days where as 0.25% of garlic oil kill fungus thoroughly within 4 days.
Antiparasitic activity:
The in vitro antiparasitic activity of diallyl trisulfide examined by Lun et al., 1993 against animal and human pathogenic protozoa Entamoeba histolytica ,Giardia lamblia and Trypanosoma sp. The result showed that diallyl trisulfide exhibited excellent activity with LC50 value range of 0.8-5.5µg/ml for different T. spp, LC50 value of 49µg/ml for E. histolytica and LC50 value of 59µg/ml for G. lamblia.
Soffar and Mokhtar 1991 studied antiparasitic effect of garlic aqueous extract hymenolepiasis nana and giardiasis. The result showed that the minimal lethal concentration which was found to be 1/20.
Anticancer activity:
Allah et al., 2015 screened anticancer potential of synthetically prepared diallyl sulfanes derivatives. The results indicated that diallyl sulfanes derivatives caused oxidative stress in cancer cells which lead to apoptosis at effective concentration of 50µg/ml.
Dvorkova et al., 2015 studied anticancer potent of allyl sulfides on both normal as well as cancer cell. The outcome indicated that Cancer cells were prone to be more sensitive towards biological immune processes like apoptosis induced by garlic compounds (allyl sulfides) in comparsion to the healthy cells of body . Diallyl disulfide caused the reduction of histone deacytylase enzyme which further leads to histone acetylation in cancer cells of rat liver and cancer cells of human breast, where as diallyl trisulfide was found to be responsible for self-death (apoptosis) in cancer cells of human prostate in comparison with diallyl disulfide.
Dong et al., 2014 studied black garlic extract for their anticancer potential. The results demonstrated that garlic extract inhibited HT29 cell growth via the induction of apoptosis and cell cycle arrest. The cell growth inhibition rate at concentrations of 100, 50 and 20 mg/ml was 46.7±4%, 40.4±5 and 24.6±4 respectively, at 24 h; 55.2±3%, 48.1±4 and 28.3±5 respectively, at 48 h; and 63.9±5%, 56.1±6 and 34.2±4 respectively, at 72 h (P<0.05).
Na et al., 2012 examined anticancer potential of diallyl trisulfide. The study indicated diallyl sulfide (DAS), diallyl disulfide (DADS), and diallyl trisulfide (DATS) all out turn to negative effects on cancerous cells. Among all sulfides DATS exhibited highest potent anti-proliferative rate in MCF-7 cells of human breast cancer.
Seki et al., 2012 studied anticancer effects of diallyl trisulfide on human colon cancer cells at 100µg/ml concentration for different interval of time indicated that diallyl trisulfide possed anticancer potent due to its sulfhydryl group which react with the cysteine group of affected cell and ultimately responsible for mitotic arrest of cancer cells. The anticancer potent of the alk(en)yl sulfides was tested using human colon cancer cells HCT-15 and DLD-1. The experimental results indicated that diallyl trisulfide suppressed the major fraction of cancerous cells followed by diallyl disulfide and diallyl monosufide., further reults indicated that diallyl trisulfide disrupted microtubule network formation of the cancer cells, and microtubule fragments could be seen at the interphase.
The anticancer effect of tetrasulfides were checked for human breast cancer cells is mediated through the inhibition of the cell division cycle 25 phosphatases by Viry et al., 2011. The result indicated that tetrasulfides appear to target different signaling pathways to inhibit cancer cell proliferation.
Nakagawa et al., 2001 studied growth inhibitory effect of diallyl disulfide on human breast cancer cell lines. The study indicated that diallyl disulfide proved to be an effective inhibitor of both ER-positive and negative human breast cancer cells and implied that diallyl disulfide caused apoptosis and DNA fragmentations in HCT-15 human colon tumor cells. The effective concentrations for diallyl disulfide found to be 1.82µM for 72 hours.
CONCLUSION
The present review literature describes the different synthetic schemes for synthesis of allyl sulfides along with their broad spectrum biological applications. The immense therpautical properties of allyl sulfides seeks scientist attention and demands their further exploration in medicinal field and agriculture field.
REFERENCES
1) Abbasi M., Mohammadizadeh R.M and Saeedi N (2016); Synthesis of symmetrical disulfides by reacting organic halides with Na2S2O35H2O in DMSO ;Commun; 40(1); 1-5
2) Allah D.R., Schwind L., Asali I.A., Nasim J., Jacob C., Gotz C and Monteranh M (2015); A scent of therapy;synthetic polysulfanes with improved physic-chemical properties induce apoptosis in human cancer cells; Int. J. Oncol; 47(3); 991-1000
3) Alli JA., Boboye BE., Okonko IO., Kolade AF., Nwanze JC (2011); In-vitro assessments of the effects of garlic (Allium sativum); extract on clinical isolates of Pseudomonas aeruginosa and Staphylococcus aureus; Adv. Appl. Sci. Res; 2(4); 25-36
4) Anastasiadis I., Kimbaris A. C., Kormpi M and Karanastasi E (2011); The effect of a garlic essential oil component and entomopathogenic nematodes on the suppression of Meloidogyne javanica on tomato; Hellenic Plant Protection Journal 4(1); 21-24
5) Anwar A., Gould E., Tinson R., Groom M and HamiltonC. J (2017); Think yellow and keep green-role of sulfanes from garlic in agriculture; Antioxidants; 6(1); 1-12
6) Archer N. J; Rayner C. M., Bell D and Miller D (1994); Synthetic routes to novel homochiral sulfenyl sulfonium salts and their use as potential enantioselective sulfenylating agents asymmetric synthesis via homochiral thiiranium ions; Synlett; 1994(8); 617-619
7) Avato P., Trusi F., Vitali C., Miccolis V and Candido V (2000); Allylsulfide constituents of garlic volatile oil as antimicrobial agents; Phytomedicine; 7(3); 239-243
8) Ayodele E. T., Olajire A. A., Oluyemi E. A and Ajanaku K. O (1999); Synthesis and fungicidal of substituted dibenzyl trisulfides; Bull. Chem. Soc, 15(1); 47-55
9) Babudari F., Farinola G. M., Fiandanese V., Mazzone L and Naso F (1998); A straightforward route to polyenylsflanes by palladium-or nickelcatalyzed cross-coupling reactions; Elsevier; 54(7); 1085-1094
10) Backvall Jan-E., Nilsson Ylva I. M., Andersson Pher G., Gatti Roberto G. P and Wu J (1994); Carbon-carbon bond formation in palladium(II);-catalyzed intramolecular 1.,4-oxidation of conjugated dienes Tetrahedron Lett; 35(31); 5713-5716
11) Bahrami K., Khodaei M.M., Khodaboustan N (2011); Tapc-catalyzed synthesis of thioethers from thiols and alcohols; Synlett; 2011(15); 2206-2210
12) Baker A., Graz M., Saunders R., Evans G. J. S; Kaul S and Wirth T (2013); Flow synthesis of symmetrical di and trisulfides using phase transfer catalysis; J., Flow., Chem; 3; 118-121
13) Banerji A and Kalena P. G (1980); A new synthesis of organic trisulfides; Tetrahedron let; 21(31); 3003-3004
14) Bates C. G., Saejueng P., Doherty M.Q and Venkataraman D (2004); Copper-catalyzed synthesis of vinyl sulfides; Org. Lett; 6(26); 5005-5008
15) Begue J P., Delpon D B and Rock M H (1996); Z-trifluoromethyl thioenol ethers., enol ethers., and enamines; reactivity towards organolithium reagents; Tetrahedron Lett; 37(2); 171-174
16) Bhaumik I; and Misra K., A; (2017); Rapid transformation of alkyl halides into symmetrical disulfides using sodium sulfide and carbon disulfide; Syn.,Open; 1(1); 0117-0120
17) Casella S., Leonardi M., Melai B., Fratini F and Pistelli L (2013); The role of diallyl sulfides and dipropyl sulfides in the In vitro antimicrobial activity of the essential oil of garlic., Allium sativum L., and Leek., Allium porrum L; Phytother., Res; 27(3); 380-383
18) Coldham I and Warren S (1993); Synthesis of cyclic amines and allylic sulfides by phenylthio migration of β-hydroxy sulfides; J. Chem. Soc. Perkin Trans; 1993(14); 1637-1656
19) Das B., Chowdary N., Damdor K and Banerjee J (2007); Efficient stereoselective synthesis of (Z);- and (E);-allyl sulfides and potent antifungal agent.,(Z);-3-(4-Methoxybenzylidene);thiochroman-4-one from Morita-Baylis-Hillman Acetates; Org. Chem. Div; 55(8); 1274-1276
20) Dong M., Yang G., Liu H., Liu X., Lin S and Sun D (2014); Aged black garlic extract inhibits HT29 colon cancer cell growth via the PI3K/Akt signaling pathway; Biomed Rep; 2(2); 250-254
21) Dvorakova M., Weingartova I., Nevoral J., Nemecek D and Krejcova T (2015); Garlic sulfur compounds suppress cancerogenesis and oxidative stress; a review; Animal., Sci., J., 46(2); 65-72
22) Gao N., Zheng S., Yang W and Zhao X (2011); Carbon-sulfur bond formation via iridium-catalyzed asymmetric allylation of aliphatic thiols; Org., Lett; 13(6); 1514-1516
23) Gara O., Hill DJ and Maslis DJ (2000); Activities of garlic oil., garlic powder and their diallyl constituents against Helicobacter pylori; Appl. Environ. Microbiol; 66(5); 2269-2273
24) Gosh K., Rajit S and Mal D (2015); A convenient method for the synthesis of aryl methyl sulfides via Cu(I);-mediated methylthiolation of haloarenes with DMSO; Tetrahedron., Lett; 56(37); 5199-5202
25) Goux C., Hoste L and Siriou D (1994); Palladum(O);-catalyzed alkylation of thiols; Tetrahedron; 50(34); 10321-10330
26) Grayson N., M and Houk K., N (2016); Cinchona urea-catalyzed asymmetric sulfa-michael reactions; The bronsted acid-hydrogen bonding model; J., Am., Chem., Soc; 138(29); 9041-9044
27) Gridnev I. D., Miyaura N and Suzuki A (1993); Convenient one-pot synthesis of vinylic sulfides from thioalkynes via a catalytic hydroboration-coupling sequence; Cheminform; 58; 5351-5354
28) Hiver P., Dicko A and Paquer D (1994); Medium effects in unsymmetrical disulfides compounds synthesis from bunte salts; Tetrahedron Lett; 35(51); 9569-9572
29) Hunter R., Caira M and Stellenboom N (2006); Inexpensive.,one-pot synthesis of unsymmetrical disulfides using 1-chlorobenzotriazole; J. Org. Chem; 71(21); 8268-8271
30) Iciek M. B., Pachel D. K., Kwiecien I and Dudek M. B (2012); Effects of different garlic‐derived allyl sulfides on peroxidative processes and anaerobic sulfur metabolism in mouse liver; Phtoether Res; 26(3); 425-431
31) Johnson M., Olaleye O. N and Kolawole O. N (2016); Antimicrobial and antioxidant properties of aqeous garlic (Allium sativum); against Staphylococcus aureus and Pseudomonas aeruginosa; Br. Microbiol. Res. J; 14(1); 1-11
32) Jonker D., Broke E. VD., Dooren I. V., Thjis C., Dorant E., Hageman G and Stobberingh E (1999); Antibacterial effect of garlic and omeprazole on Helicobacter pylori; J. Antimicrob. Chemother; 43(6); 837-839
33) Kanemoto K., Sugimura Y., Shimizu S., Yoshida S and Hosoya T; (2017); Rhodium-catalyzed odorless synthesis of diaryl sulfides from borylarenes and S-aryl thiosulfonates; Chem Commun; 53(77); 10640-10643
34) Kertmen A., Lach S., Rachon J and Witt D (2009); Novel and efficient methods for the synthesis of symmetrical trisulfides; Synthesis; 2009 (9); 1459-1462
35) Kondo T., Morisaki Y., Uenoyama S., Wada K and Mitsudo T (1999); First ruthenium-catalyzed allylation of thiols enables the general synthesis of allylic sulfides; J. Am. Chem. Soc; 121(37); 8657-8658
36) Slawoir L ., Kasyzynksa M. S and Witt D (2010); A novel and efficient synthesis of unsymmetrical trisulfides; Synlet; 2010(19); 2857-2860
37) Lee H., P., Park Y., Lee E and Kim S (2011); Synthesis of di., tri and tetrasulfides through multifold carbon-sulfur cross-coupling reactions with indium tri(organothiolates); in a one-pot procedure; J., Org., Chem; 76(3); 760-765
38) Leuschner R G and Ilesh V (2003); Antimicrobial effects of garlic., clove and red hot chilli on Listeria monocytogenes in broth model systems and soft cheese; Int. J. Food Sci. Nutr; 54(2); 127-133
39) Li G., Ma X., Deng L., Zhao X., Wei Y; Gao Z; Jia J; Xu J and Sun C (2015); Fresh garlic extract enhances the antimicrobial activities of antibiotics on resistant strains in vitro; Jundishapur J Microbiol; 8(5); 4814-4815
40) Li WR., Shi QS., Dai HQ., Liang Q., Xie XB., Huang XM., Zhao GZ and Zhang LX (2015); Antifungal activity., kinetics and molecular mechanism of action of garlic oil against Candida albicans; Sci. Rep; 6; 1-9
41) Li RW., Shi QS., Liang Q., Huang XM and Chen YB (2014); Antifungal effect and mechanism of garlic oil on Penicillium funiculosum; Appl. Micro. Biotechnol; 98(19); 8331-8346
42) Liu S., Sun Y., Li W., Yu H., Li X., Liu Z., Zeng J., Chen C and Jia J (2010); The antibacterial mode of action of allitridi for its potential use as a therapeutic agent against Helicobacter pylori infection; Microbiol Lett; 303(2); 183-189
43) Lun ZR., Fang K, Wang CJ and Brun R (1993); Trypanosomiasis of domestic animals in China; Parasitol Today; 9(2); 41-45
44) Mashkina A V (2011); New method of dimethyl sulfide synthesis; J. Org. Chem; 47(5); 678-681
45) Meijer J and Vermeer P (1974); Synthesis of 1-(methylthio);propyl propenyl disulfide and sec-butyl 3-(methylthio);allyl disulfide., two pesticide from Asa foetida; Recueil des Travaux Chimiques des Pays-Bas; 19; 2857-2860
46) Meshram HM (1993); An efficient and mild cleavage of thiol acetate with clayfen in the absence of solvent; Tetrahedron Lett; 34(15); 2521-2522.
47) Yu M and Zhang Y (1997); The synthesis of allyl sulfides by organosamarium reagent; Synth Commun; 27(16); 2743-2748
48) Miranda E I., Diaz M J., Rosario I and Soderquist J A (1994); Thiols., unsymmetrical sulfides and thioacetals from the new reagent; triisopropylsilanethiol; Tetrahedron Lett; 35(20); 3221-3224
49) Mnayer D., Tixier F. AS., Petitcolas E., Hamich T., Nehme N., Ferrant C., Fernandez X and Chemat F (2014); Chemical composition., antibacterial and antioxidant activities of six essential oils from Alliaceae Family; Molecules; 19(12); 20034-20053
50) Mohammadi F and Beigi M (2016); Simple and green method for synthesis of symmetrical dialkyl disulfides and trisulfides from alkyl halides in water; PMOxT as a sulfur donor; J. Sulfur. Chem; 38(2); 1-8
51) Moore C. G and Trego B. R (1963); The reaction of triphenylphosphine with unsymmetrical dialkenyl disulfides and with symmetrical dialkenyl trisulfides; Tetrahedron; 19(8); 1251-1258
52) Na HK., Kim EH., Choi MA., Park JM., Kim DH and Surh YJ (2012); Diallyl trisulfide induces apoptosis in human breast cancer cells through ROS-mediated activation of JNK and AP-1; Biochem. Pharmacol; 84(10); 1241-1250
53) Nakabayashi T and Tsurugi J (1961); Organic polysulfides.III.synthesis and some properties of several unsymmetrical polysulfides; J. Org. Chem; 26(7); 2482-2486
54) Nakagawa H., Tsuta K., Kiuchi K., Senazaki H., Tanka K., Hioki K and Tsubura A (2001); Growth inhibitory effects of diallyl disulfides on human breast cancer cell lines; Carcinogenesis; 22(6); 891-897
55) Nishimoto Y., Okita A., Yasuda M and Baba A (2012); Synthesis of a wide range of thioethers by indium triiodide catalyzed direct coupling between alkyl acetates and thiosilanes; Org., Lett; 14(7); 1846-1849
56) Ogawa A., Ikeda T., Kimura K., and Hirao T (1999); Highly regio- and stereocontrolled synthesis of vinyl sulfides viatransition-metal-catalyzed hydrothiolation of alkynes with thiols; J. Am. Chem. Soc; 121(22); 5108-5114
57) Oswald A. A., Griesbaum K and Hall D. N (1968); Synthesis of terminally difunctional polythioethers by polyaddition reactions involving allylic sulfides; J. Polymer Sci; 24(1); 113-123
58) Oueslati Y., Abidi A., Sbihi H., M and Rezgui F; (2016); A direct synthetic route to allyl sulfides from cyclic Morita–Baylis–Hillman alcohols; J. Sulfur Chem; 38(2); 142-151
59) Park IK., Kim J; Lee SG and Shin SC (2007); Nematicidal activity of plant essential oils and components from garlic (Allium sativum); and cinnamon (Cinnamomum verum); oils against the pine wood nematode (Bursaphelenchus xylophilus); J.Nematol; 39(3); 275-279
60) Prakash S. G. K., Hoole D., Ha S. D., Wilkinson J and Olah A G (2002); Convenient preparations of aryl methyl and aryl phenyl sulfides from arenediazonium tetrafluroborates and trimethyl(methylthio); and trimethyl(phenylthio);-silanes; Arkivoc; 2002(13); 50-54
61) Qiao Q., Dominique R., Sidduri A., Lou J and Goodnow A. R (2011); Efficient synthesis of aryl methyl sulfide derivatives using (methylthio);trimethylsilane as methylthiolation reagent; Synth. Commun; 40(24); 3691-3698
62) Rattanachaikunsopon P and Phumkhachorn P (2008); Diallyl sulfide content and antimicrobial activity against food-borne pathogenic bacteria of chives (Allium schoenoprasum); 72(11); 2987-2991
63) Rattanachaikunsopon P and Phumkhachorn P (2009); Antimicrobial activity of elephant garlic oil against Vibrio cholerain vitro and in a food model; Biosci. Biotechnol. Biochem; 73(7); 1623-1627
64) Ren FK., He XY., Deng Li., Li BH ., Shin DS and Li ZB (2009); Synthesis and antibacterial activity of 1.,3-diallyltrisulfane derivatives; Bull. Korean Chem. Soc; 30(3); 687-690
65) Richter L S., Marsters J. C., Gadek T. R (1994); Two new procedures for the introduction of benzyl-type protecting groups for thiols; Tetrahedron Lett; 35(11); 1631-1634
66) Rivlin S., R., (2001); Historical perspective on the use of garlic; J. Nutr; 131(3); 951-954
67) Ross ZM., O, Gara EA., Hill DJ., Sleightholme HV and Maslin DJ (2001); Antimicrobial properties of garlic oil against human enteric bacteria; evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder; Appl. Environ. Microbiol; 67(1); 475-480
68) Nava-Salgado VO; Peters EM; Peters K; von-schnering HG and Adam W (1995); The diastereoselective synthesis of ester-functionalized alkenes through the stereocontrolled conjugate addition (Michael reaction); of prochiral enolates to chiral α-methylene β-lactones and thermal decarboxylation of the resulting α-substituted β-lactones; J. Org. Chem; 60(12); 3879-3886
69) Seki T., Hosono T., Suda S., Kimura K and Ariga T (2012); Anticancer property of allyl sulfides derived from garlic J. Food Drug Anal; 20(1); 309-312
70) Selvaraj V and Rajendran V (2016); Synthesis of diallyl thioether under influence of ultrasound assisted multi-site phase transfer catalysis condition- a kinetic study; IJRD; 12(8); 29-37
71) Sinha P., Kharagpur and Roy S (2003); Process for the preparation of diorganotrisulfide; US Patent 6555712
72) Soffar SA and Mokhtar GM (1991); Evaluation of the antiparasitic effect of aqueous garlic (Allium sativum); extract in Hymenolepiasis nana and giardiasis; J Egypt Soc Parasito; 21(2); 497-502
73) Soltan R H., Ahmed M S and Emam A D (2016); Comparative antibacterial activity of garlic essential oil extracted by hydro-distillation and diethyl ether extraction methods on four pathogenic bacteria; Adv. Plants. Agric. Res; 4(2); 1-5
74) Toohey J. I. (1989); Sulphane sulphur in biological systems: A possible regulatory role; Biochem. J; 264(3); 625–632
75) Tsao SM and Yin MC (2001); In vitro antimicrobial activity of four diallyl sulfides occurring naturally in garlic and Chinese leek oils; J. Med. Microbiol; 50(7); 646-649
76) Venkatesh Y. P. ( 2018) ; Immunomodulatory attributes of aged garlic extract and its components; Immunology; 1; 203-224
77) Viry E., Anwar A., Kirsch G., Jacob C., Diederich M and Bagrel D (2011); Antiproliferative effect of natural tetrasulfides in human breast cancer cells is mediated through the inhibition of the cell division cycle 25 phosphatases; Int. J. Oncol; 38(4); 103-111
78) Wang HC., Pao J., Lin SY and Sheen LY (2012); Molecular mechanisms of garlic-derived allyl sulfides in the inhibition of skin cancer progression; New York Academy of Sciences; 1271 (1); 44-52
79) Wang K., Groom M., Sheridan R., Zhang S and Block E (2013); Liquid sulfur as a reagent; synthesis of polysulfanes with 20 or more sulfur atoms with characterization by UPLC-(Ag+);-coordination ion spray-MS; J. sulfur chem; 34(1-2); 55-66
80) Wu X., Lou J and Yan G (2016); Metal-free dtbp-mediated methylthiolation of arylboronic acids with dimethyldisulfide; Synlett; 27; 2269-2273
81) Wu X., Rieke R. D and Zhu L (1996); Preparation of disulfides by oxidation of thiols using bromine; Synt Commun; 26(1); 191-196
82) Yatusmonji Y., Ishida Y., Tsubouchi A and Takeda T (2007); Nickel(0); triethyl phosphite complex-catalyzed allylic substitution with retention of regio- and stereochemistry; Org. Lett; 9(22); 4603-4606
83) Zhan Z., Lu G and Zhang Y (1999); A novel synthesis of allyl sulfides and allyl selenides via Sm-BiCl3 system in aqueous media; J. Chem. Res; 49(4); 280-281
84) Zhan Z.P and Lang K (2004); One-pot synthetic method of allyl sulfides: samarium-induced allyl bromide mediated reduction of alkyl thiocyanates and diaryl disulfides in methanolic medium; Chem. Lett; 33(10); 1370-1371.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









