 About Authors:
About Authors:
Dhiren Shah
m.pharmacy
Seth G.L.Bihani S.D.College of Pharmacy, R.U.H.S.
Sri Ganganagar, Rajasthan, INDIA
dhiren.pharmacist@gmail.com
Abstract
A method has been developed for immobilisation of antisera on fresh plastic tubes through an immunochemical bridge. This type of immobilisation has been shown to be more consistent than direct adsorption on plastic. Such immunochemically coated antisera on plastic tube has been used in the development of a noncentrifugation radioimmunoassay. This assay system has been found to be technically as sound as the conventional method.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1489
INTRODUCTION
Radioimmunoassay (RIA) is a laboratory method that measures, with relative accuracy, minute amounts of substances present in the body. When it was developed in the 1950’s, radioimmunoassay was considered a revolution in medical investigations. As of 2009, it is still considered revolutionary because it is the blueprint for more advanced methods of laboratory techniques.
First discovered by Rosalyn Yalow and Solomon Berson, radioimmunoassay was used to investigate blood volume, iodine metabolism, and hormones like insulin. Radioimmunoassay has expanded its viability by being able to measure trace amounts of substances using sensitive laboratory techniques. Drugs, antigens and hormones are some of the substances measured by radioimmunoassay.
Radioimmunoassay is a specific scientific process. First, laboratory technicians must obtain a substance that contains the antigen they are testing for. This antigen is then injected with radioactive chemicals, such as a gamma-radioactive isotope made from iodine or some other substance. The radioactive chemicals cause the antigen to become radioactive, so it can be observed.
[adsense:468x15:2204050025]
The radioactive antigen is then mixed with a set amount of antibodies that scientists have determined are appropriate. The antigens and antibodies bind to each other and become one substance. This provides the benchmark or basis for testing. Then, an unknown substance which contains some tiny amount of the antigen is added. This new substance is the substance being tested.( wisegeek.com/what-is-radioimmunoassay.htm)
Immunoanalytical methods that are based on the selective, reversible binding of small molecules (drugs) or macromolecules by biologically derived antibodies have revolutionized the ?eld of biomedical analysis. Immunoassays have allowed the determinaion of very small amounts of analytes that were previously unassayable in biological matrices by other techniques. Since the origin al work on the analys is of insulin by Berson and Yalow.
Immunoassay methods have been developed for the determination of a wide variety of drugs, pesticides, hormones, and biological proteins. Immunoassays are relatively simple procedurally. This has led to the development of many ‘‘kit-type’’ immunoassay systems that are used routinely for home diagnostics. Anti bodies that are used as reagents in immunoassays are, in general, molecules of the immunoglobulin G (IgG) type. They are produced by white blood cells in response to foreign substances introduced in mammalian species. Million s of years of vertebrate evolution have developed immunoglobulins into exquisitely discriminating devices capable of recognizing subtle differences between molecules; for example, a mouse can generate millions of different immunoglobulin speci?cities. These immunoglobulins combine speci?cally with the substances (antigens) that elicited their formation. This then triggers processes by which the foreign antigens are cleared from the organism, which is the ultimate goal of the immune process.(Dr.Arora.D.R)
These molecules are heterogeneous, bifunctional glycoproteins in which the variable amino acid sequence in the polypeptide component provides its biologic activity. This polypeptide component is made up of two heavy or H chains (50,00 0 Da) and two light or L chains (20,000 Da), held together by disul?de bonds . The two binding sites of the antibody molecule appear to reside on the NH terminal ends of the polypeptide chains.
Antibodies produced by injection of foreign antigens into a host animal are structurally heterogeneous and respond to different aspects of the same antigen with different binding strengths and speci?cities. If the antibody -producing blood cell can be isolated in a pure cell culture, however, only one particular antibody structure will be produced.
Antibodies harvested from pure cell cultures are referred to as monoclonal antibodies, are homogeneous and react with only one or a few closely related anti- gens. The antibody producing cells which can be grown in culture are produced by cell fusion techniques and provide superior antibodies for use in immunoassays because of their identical speci?cities and binding strengths. Antigens must have molecular weights in excess of 104 Da in order to evoke antibody production. However, small molecules, such as drugs, can be bound to macromolecular carriers and some of the antibodies produced from these will respond to the drug (hapten).
All immunoassay procedures take advantage of the speci?c reactions between antibodies an d antigens. They involve measurement, directly or indirectly, of the extent of binding between antibodies (reagents) and antigens (analytes). Labels are used in conjunction with the antigens or antibodies so that the concentrations of molecular species can be measured instrumentally. Labels are entities that impart some measurable signal, such as radioactivity, ?uorescence, chemiluminescence, on electrochemical or enzyme activity to the antibody or antigen to which it is attached. The determination of the extent of antibody binding requires measurements of the amounts of labeled antigen or antibody in the complexed (bound) and in the free forms. This is generally expressed as the bound/ free (b/f ) concentration ratio, which is related to the concentration of analyte. The measured signal can be either directly or inversely proportional to the b/f ratio depending on the chemistry of the system used. There are two ways to determine the amount of antigen present: one using a limited amount of reagent (competitive assays), the other using an excess amount of reagent (non-competitive assays).
The competitive assay uses a limited amount of antibody, which is insuf?cient to bind all of the antigen. The antigen competes with a ?xed amount of labeled antigen for the limited number of antibody binding sites. From the proportion of bound (or free) labeled antigen, the concentration of unlabeled antigen can be determined.
The non-competitive assay uses an excess of anti-body. Different approaches to detect the bound antigen have been developed, the most common use an antibody , in excess , coupled to a solid phase. The bound antigen is then detected with a second antibody labeled in a way that aids detection (e.g., radioactive , ?uorophore, etc.) . The amount of antigen in the sample is then directly proportional to the amount of labeled antibody captured on the solid phase. Both assays require differentiation of bound label from free label. This can be achieved by two methods: heterogeneous assay or homogeneous assay. Heterogeneous assay separates bound label from free label using a means of removing the antibody. Homogenous assay compares the signal of the label when antigen is bound to antibody compared to when the antigen is free.(discoveriesinmedicine.com/Ni-Ra/Radioimmunoassay-RIA.html)
Principle
The principle involves competitive binding of radiolabeled Ag and unlabeled Ag to the limited supply of a high affinity Ab.
Immunoanalytical methods that are based on the selective, reversible binding of small molecules (drugs) or macromolecules by biologically derived antibodies have revolutionized the ?eld of biomedical analysis.(Kamboj.P.C)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Advantages & Disadvantages of RIA
Advantages
– Highly specific: Immune reactions are specific
– High sensitivity : Immune reactions are sensitive
Disadvantages
– Radiation hazards: Uses radiolabelled reagents
– Requires specially trained persons
– Labs require special license to handle radioactive material
– Requires special arrangements for
• Requisition, storage of radioactive material
• radioactive waste disposal.
Antibodies
• Antibodies are produced by the B lymphocytes. They are glycoproteins belonging to the “immunoglobulin supergene family” (Wild) that are produced in response to a foreign substance in the body.
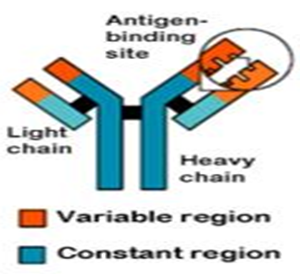
Figure 1:- Antigen binding site
• Antibodies have a generally common structure, but have regions that vary among them to accommodate the specific antigens.
Antigens
• An antigen is a substance with the ability to induce an immunological response. They typically enter the body from an infection. They are recognized at their epitopes by B cells or by the T cell receptor on T cells.
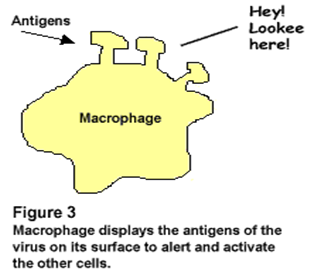
• Proteins or glycoproteins make the best antigens because they are the best at stimulating antigen recognition molecules. Some immunoassays test for antigens, rather than antibodies.
Production of Antibodies
• The production of antibodies is an important process in the use of immunoassays because it is the antibody-antigen complexes that form that the device uses for its results. Antibodies can be called monoclonal or polyclonal, depending upon the technique used to produce it.
• Monoclonal antibodies involve these basic steps and result in very specific antibodies that bind only to one antigen epitope, which in turn reduces the occurrence of false positives in the immunoassay:
− A mouse (or rabbit) is immunized by being injected with an antigen; the antigen generates an antibody response in the animal.
− The mouse is sacrificed and the antibody forming cells are isolated from the mouse's spleen.
− Monoclonal antibodies are produced by fusing single antibody-forming cells with myeloma cells. The resulting cell is called a hybridoma. This hybridization makes the cells “immortal.”
The hybridomas contain large amounts of antibodies, and can easily be cultured into populations of cells that contain identical antibodies. These antibodies are called "monoclonal antibodies" because they are produced by the identical offspring of a single, cloned antibody producing cell. (Glick.Bnard.R)
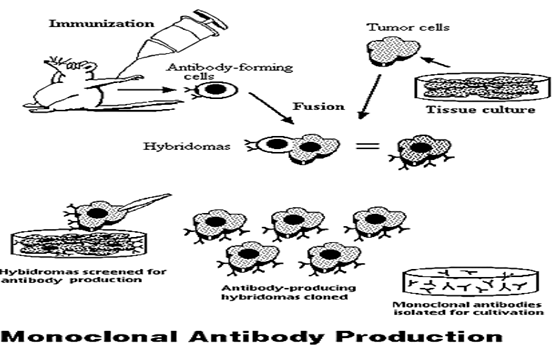
Figure 3
A diagram depicting the steps involved in monoclonal antibody production.
Production of Antibodies Cont’d
• Polyconal antibodies are more likely to produce a false positive because they are less specific to antigen epitopes and have varying binding affinities; they may bind of molecules similar to their antigens. Production includes these steps:

Figure 4 :- Inject Antigen and Sample blood
− A mouse (or rabbit) is immunized by being injected with an antigen; the antigen generates an antibody response in the animal.
− The animal is bled and the antibodies are collected; the blood contains a heterogenous mixture of antibodies of varying binding affinities and specificities.
The main drawbacks to radioimmunoassay are the expense and hazards of preparing and handling the radioactive antigen.
- Both 125I or 131I emit gamma radiation that requires special counting equipment;
- The body concentrates iodine atoms — radioactive or not — in the thyroid gland where they are incorporated in thyroxine (T4).
INSTRUCTIONS RELATING TO THE HANDLING, USE, STORAGE, AND DISPOSAL OF THIS RADIOACTIVE MATERIAL
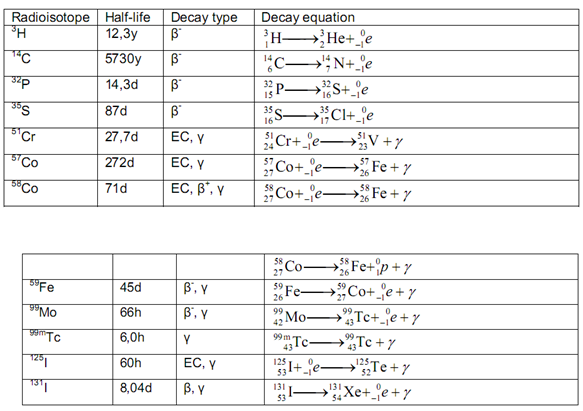
Table 1 :- Radio Active Material
( RADIOIMMUNOASSAY LABORATORY)
• This radioactive material may be received, acquired, possessed, and used only by research laboratories for in vitro laboratory tests not involving internal or external administration of the material, or the radiation therefrom, to human beings or animals. Its receipt, acquisition, possession, use and transfer are subject to the regulations and a general license of the U. S. Nuclear Regulatory Commission or of a State with which the Commission has entered into an agreement for the exercise of regulatory authority.
1. All radioactive materials must be labeled and secured in specifically designated posted areas. Records of receipt and survey must be maintained.
2. All work with these materials must be carried out only in authorized areas.
3. Prohibit mouth pipetting of radioactive materials.
4. There must be no smoking or eating within the work area.
5. Hands must be washed after handling radioactive materials.
6. Any spilled material must be wiped up quickly and thoroughly and the
• contaminated substances transferred to a suitable receptacle. The surfaces involved must be washed thoroughly with an appropriate decontaminant. Monitor to ensure the area has been effectively decontaminated.
7. When use of the Tracer reagent has been completed, empty and decontaminate the vial. This radioactive material can be discarded into the sanitary sewerage system using copious amounts of water to ensure a minimal discharge concentration.
8. Prior to disposal of the empty, uncontaminated Kit and Tracer containers to unrestricted areas, remove or deface the radioactive material labels or otherwise clearly indicate that the containers no longer contain radioactive material. (Ananthanarayan.R)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Labels in Immunoassays
Immunoassays require the use of labeled materials in order to measure the amount of antigen or antibody present. A label is a molecule that will react as part of the assay, and in doing so produce a signal that can be measured in the solution. Examples of a label include a radioactive compound, or an enzyme that causes a change of color in a solution or its fluorescence. (Vyas.S.P)
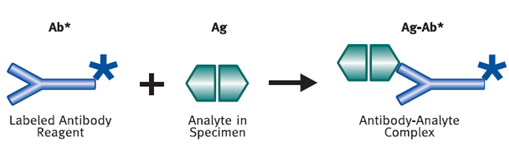
Figure 5 :- Antibody-Analyte Complex
RADIOLABELEDIMMUNO ASSAYS
The earliest immunoassays made use of radioactively labeled antigens or antibodies. These analytes are referred to as radiolabeled immunoassays . Antibody binding sites are extremely speci?c and to retain this speci?ty the best option would be to replace a non- radioactive isotope in the tracer molecule by its radioisotope (e.g., replace hydro gen by 3H). However when the substitution is made in a part of the molecule away from the antibody binding site, the choice of radio-isotope can be governed by other considerations, such as half-life, availability, high activity, and radiochemical purity .
The most common radioactive label is 125I, which is bound to antibodies and antigens by a variety of techniques including chloramine- T, iodogen, or lactoperoxidase iodination. Iodination with 125I is the preferred radiolabeling technique be cause the isotope is a g -emitter, inexpensive, can be easily detected , has an appropriate half- life for most analytical purposes, and can be obtained in preparations with high speci?c activity. Problems occasionally associated with radioiodination include loss of immunoreactivity due to the size of the label or chemical alteration of reagent s due to the high energy of decay.
Use of beta-emittin g isotopes (14C and3H) may resolve these problems, but require more complicated scintillation counting for label measurement . (Encyclopedia)
Labeled Antigen Radioimmunoassays
Labeled antigen radioimmunoassays involve competition between a labeled antigen and an unlabeled antigen (analyte) for a limited number of antibody combining sites as shown in the following reactions:
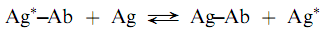
where Ag is labeled reagent antigen, Ag is analyte antigen, and Ab is antibody against Ag. The Ag and Ab are analytical reagents with concentrations ?xed so that when a sample containing Ag is added, competition between Ag and Ag for Ab binding sites occurs. Increasing concentrations of Ag result in a lesser degree of binding of Ag and the measurement of radioactivity of the binding or free fraction can be used to determine the amount of Ag present in a sample.
Determination of the distribution of Ag between the antibody -bound and free fractions , in radioimmunoassay procedures, requires a separation step which isolates Ag from Ag–Ab. Once separated, measurement of the radioactivity of the label in one or both the fractions provides a signal that is approximately exponentially related to the concentration of unlabeled antigen. The graphical relationship can have either a positive or a negative slope depending on whether the antibody -bound or free fraction is measured. The exact shape of the calibration curve however, is dependent on the antibody -binding equilibrium constant.
A variety of methods has been applied to the separation of bound and free Ag. These include precipitation, solid phase attachment, capillary electrophoresis, chromatography, and micro?ltration. Originally, precipitation and solid-phase extraction were the most common types of separations techniques. However the ease of automation of capillary electrophoresis and ?ow-injection analysis (chromatography) makes these two techniques very interesting.
Precipitation
Precipitation techniques can be categorized into two general classes, non-speci?c and speci?c. The non-speci?c separations involve the addition of a salt or solvent that decreases the solubility of the antigen– antibody complex under conditions that do not affect the free-labeled antigen. After addition, the immune complexes can be precipitated by centrifugation and the radioactivity in either the supernatant solution or the precipitate can be measured. Example s of precipitation reagents used in immunoassays include alcohol, ammonium sulfate, polyethylene, and dioxane. Care must be taken to avoid co precipitation of the unbound label.
Speci?c precipitation is a technique that has been devised to overcome some of the problems associated with non-speci?c techniques. In this approach, a second antibody (Ab0), speci?c to the analytical antibody is added in excess to cause precipitation of the primary antigen–antibody complex as shown below:
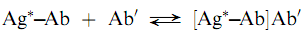
The method has been termed the double-antibody technique and can be used for a wide variety of analytes. The double antibody techniques generally require more time because the second antibody reaction can require days to reach equilibrium . The speed of precipitation clearly depends on the concentration of the second antibody . Polyethylene glycol has been used as a cosolvent to increase the precipitation rate. (Saha.Gopal.B)
Solid phase techniques
Many early immunoassays used solid phases to separate the labeled antigen from the complex by differential adsorption of the former. Examples of the solid phases uses are dextran-coated charcoal , ion-exchange resin s, and cellulose powder . Once the labeled antigen has been adsorbed, the bound, labeled antigen could be de canted to allow the measurement of the radioactivity of the bound and/o r free-labeled material sseparately. Because of problems with non-speci?c adsorption and errors due to stripping of the labeled antigen from the solid, these types of separations have been largely replaced by those employing solid phases in which the reagent is covalently bound to the solid, as for example, in the solid phase attachment of a speci?c antibody (AbSP), which binds labeled and unlabeled antigen competitively as shown below: (Encyclopedia)
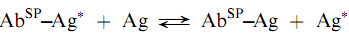
The solid phase to which the antibody is bound can be either suspended in solution, on particles such as cellulose, agarose, or dextran beads or can be attached to the surface of a test tube or a micro titer plate. The stationary solid phases eliminate the centrifugation step that is necessary with the suspended beads although plastic solid phases have a limited capacity of binding proteins.
Capillary electrophoresis
This technique has proven to be a powerful separation technique for the separation of macromolecules, such as antibodies, for two reasons: the near ?at plug ?ow pro?le and the small diffusion constant of the anti- bodies. These characteristics eliminate band broadening. With both superior separation power and high detection sensitivity, capillary electrophoresis (CE) can separate free Ab and Ag from bound Ab and Ag rapidly and is suitable for immunoassays. CE can combine immunologic recognition with online quantitation, microscale analysis, and automatic instrumentation to offer unique advantages for immunoassays. In immunoassays CE can be coupled to all of the existing CE detection techniques from UV and laser induced ?uorescence (LIF) to mass spectrometry (MS). CE coupled to LIF has been applied to the determination of a variety of compounds including therapeutic drugs, peptides, and antibodies.
Flow -injection analysis
This technique introduces a sample into a ?ow of reagents. The reaction proceeds during transport in a reactor , after which the product is measured down- stream in a detector. By combining immunoassays with ?ow-injection analysis (FIA), the long incubation times usually associated with heterogeneous assays become irrelevant, since FIA exploits non-equilibrium conditions. The was hing steps are performed automatically by the continuous ?ow of buffer. Be sides the irrelevance of incubation times, another advantage over normal immunoassays is that the reactor can be used several times because regeneration by using a suitable agent (e.g., glycine HCl) is possible. Sequential injections can be used to deliver reagents, substrate (if necessary), and regeneration agents to the immunoreactor. Many different assays using electrochemical, ?uorescent and chemiluminescent detection have been developed.
Labeled Antibody Radioimmunoassays (Immunoradiometric Assays)
The problem of binding proteins to solid phases can be avoided by employing a solid phase antigen (AgSP) and a labeled antibody (Ab) as shown in the following reactions:
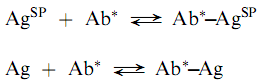
Where the unlabeled antigen (the analyte) competes for antibody binding sites with the solid phase antigen. This approach may have some difficulties caused by the requirement for puri?cation of the immunospeci?c antibody. Because monoclonal antibodies are produced as essentially immunospeci?cally pure populations, they are ideal for labeled antibody techniques.
Another approach that utilizes labeled antibodies is the so-called ‘‘sandwich technique.’’ In this method, the analyte antigen is bound both by an antibody that is attached to a solid phase (AbSP) an d a labeled antibody as follows:
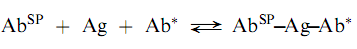
The reaction is non-competitive and thus, the concentration of unlabeled antigen has a direct relationship with the amount of label bound in the complex. The sandwich radioimmunoassays are both sensitive and convenient but are limited to analytes which are, at least, bivalent; i.e., antigens that can provide at least two sites for antibody attachment. Also, highly puri?ed antibodies are required for the sandwich technique. (Encyclopedia)
Radioimmunoassay (RIA)
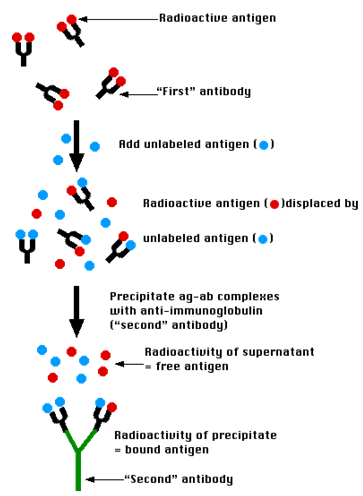
Figure 6 :- Process of the RIA Activity
The technique of radioimmunoassay has revolutionized research and clinical practice in many areas, e.g.,
- blood banking
- diagnosis of allergies
- endocrinology
The technique was introduced in 1960 by Berson and Yalow as an assay for the concentration of insulin in plasma. It represented the first time that hormone levels in the blood could be detected by an in vitro assay. (PerkinElmer Life and Analytical Sciences)
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
The Technique
A mixture is prepared of radioactive antigen. Because of the ease with which iodine atoms can be introduced into tyrosine residues in a protein, the radioactive isotopes 125I or131I are often used. Antibodies ("First" antibody) against that antigen.
- Known amounts of unlabeled ("cold") antigen are added to samples of the mixture. These compete for the binding sites of the antibodies.
- At increasing concentrations of unlabeled antigen, an increasing amount of radioactive antigen is displaced from the antibody molecules.
- The antibody-bound antigen is separated (see below) from the free antigen in the supernatant fluid, and
- The radioactivity of each is measured.
- From these data, a standard binding curve, like this one shown in red, can be drawn.
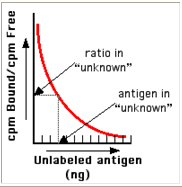
Figure 7 :- Bound Vs Unlabeled antigen
* The samples to be assayed (the unknowns) are run in parallel.
* After determining the ratio of bound to free antigen ("cpm Bound/cpm Free") in each unknown, the antigen concentrations can be read directly from the standard curve (as shown above)
Separating Bound from Free Antigen
There are several ways of doing this.
Precipitate the antigen-antibody complexes by adding a "second" antibody directed against the first. For example, if a rabbit IgG is used to bind the antigen, the complex can be precipitated by adding an antirabbit-IgG antiserum (e.g., raised by immunizing a goat with rabbit IgG). This is the method shown in the diagram above.
The antigen-specific antibodies can be coupled to the inner walls of a test tube. After incubation,
The contents ("free") are removed;
The tube is washed ("bound"), and
The radioactive of both is measured.
The antigen-specific antibodies can be coupled to particles, like Sephadex. Centrifugation of the reaction mixture separates The bound counts (in the pellet) from The free counts in the supernatant fluid.
Radioimmunoassay is widely-used because of its great sensitivity. Using antibodies of high affinity (K0 = 108–1011 M−1), it is possible to detect a few picograms (10−12 g) of antigen in the tube.
The greater the specificity of the antiserum, the greater the specificity of the assay.
The main drawbacks to radioimmunoassay are the expense and hazards of preparing and handling the radioactive antigen.
- Both 125I or 131I emit gamma radiation that requires special counting equipment;
- The body concentrates iodine atoms — radioactive or not — in the thyroid gland where they are incorporated in thyroxine (T4).(radioimmunoassay)
Applications of Radioimmunoassays
# Endocrinology
* Insulin, HCG, Vasopressin
* Detects Endocrine Disorders
* Physiology of Endocrine Function
# Pharmacology
* Morphine
* Detect Drug Abuse or Drug Poisoning
* Study Drug Kinetics
# Epidemiology
* Hepatitis B
# Clinical Immunology
* Antibodies for Inhalant Allergens
* Allergy Diagnosis
# Oncology
* Carcinoembryonic Antigen
* Early Cancer Detection and Diagnosis(Saha.Gopal.B)
Result and discussion
RIA is a very simple and easy going technique. It take very less time than any other method this is a very good and very popular method for hormone testing and pregency testing.
A method has been developed for immobilisation of antisera on fresh plastic tubes through an immunochemical bridge. This type of immobilisation has been shown to be more consistent than direct adsorption on plastic. Such immunochemically coated antisera on plastic tube has been used in the development of a noncentrifugation radioimmunoassay. This assay system has been found to be technically as sound as the conventional method.
Discussion
* Based on Simple Biochemistry Principles
* Establish Ideal Binder and Ligand
* Synthesize Tracer Ligand
* Separation of Bound and Free Parts
* High Precision and Sensitivity
* Powerful Applications to a Wide Range of Medical Fields
List of Table
|
Sr. No. |
Name of Table |
Page. No. |
|
1 |
Radio Active Material |
7 |
List of Figure
|
Sr. No. |
Name of Figure |
Page. No. |
|
1 |
Antigen binding site |
4 |
|
2 |
Macrophage displays the antigen of the virus on its surface to alert and activate the other cells. |
5 |
|
3 |
Monoclonal Antibody Production |
6 |
|
4 |
Inject Antigen and sample blood |
6 |
|
5 |
Antibody-Analyte Complex |
8 |
|
6 |
Process of the RIA Activity |
13 |
|
7 |
Bound Vs Unlabeled antigen |
14 |
REFERENCE
1) Ananthanarayan.R. et al “Textbook of Microbiology”, Published by Orient Longman. Page no.99-100
2) Dr.Arora.D.R. “Textbook of Microbiology”, CBS Publishers and Distributors, New Delhi. Page no.111-112
3) “Encylopedia of pharmaceutical technology”, Second edition, volume-2, edited by james swarbrick et al. Page no.1514-1528
4) Glick.Bernard.R. “Molecular Biotechnologh”, Third edition, Published by ASM Press, Washington. Page no.230-231
5) Kamboj.P.C. “Pharmaceutical Analysis”, Volume-2, Published by Vallabh prakashan. Page no.442-444
6) Saha.Gopal.B. “Fundamentals of Nuclear pharmacy”, Third edition, Published by Springer-Verlag. Pagen no.219-226
7) Vyas.S.P. “Method in Biotechnologh and Bioengineering”, CBS Publishers and distributors, New Delhi. Page no.74-76
8) “Pharma Times” Volume 41, No.6, June 2009, Official Publication of The Indian pharmaceutical Association. Page no. 1-4
9) Access On: 1st Aug, 2011 wisegeek.com/what-is-radioimmunoassay.htm
10) Access On: 1st Aug, 2011 discoveriesinmedicine.com/Ni-Ra/Radioimmunoassay-RIA.html
11) Access On: 1st Aug, 2011 biotech.com//week 9 Radioimmunoassay-RIA.html
12) Access On: 4th Aug, 2011 PerkinElmer Life and Analytical Sciences, Inc.
13) Access On: 4th Aug, 2011 David Haarburger, “RADIOIMMUNOASSAY LABORATORY: COMPETENCY ASSESSMENT 2008”, February 2008.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









