{ DOWNLOAD AS PDF }
ABOUT AUTHORS
Manohar D. Kengar*, Jameer A. Tamboli, Chandrakant S. Magdum
Rajarambapu College of Pharmacy, Kasegaon, Dist – Sangli, Maharashtra, India
ABSTRACT
Pharmaceutical Quality by Design (QBD) is a systematic approach to development that begins with predetermined objectives and emphasizes the understanding of production and processes and process control, based on sound science and quality risk management. Quality purchasing design (QBD) is emerging to increase the promise of providing safe and effective medicines to customers and promises to improve the efficiency of product quality. Quality means eligibility for use. The quality of the medication means that the product provides therapeutic benefits to the label reproducible and free from contamination on the label. In vivo or in vitro performance test can be evaluated for drug production. Dietite by Quality guarantees the performance of the product in vitro and the performance of the in vitro product in the in vivo product. "So the quality design is related to the product."
Reference Id: PHARMATUTOR-ART-2659
|
PharmaTutor (Print-ISSN: 2394 - 6679; e-ISSN: 2347 - 7881) Volume 7, Issue 04 Received On: 14/03/2019; Accepted On: 18/03/2019; Published On: 01/04/2019 How to cite this article: Kengar, M., Tamboli, J. and Magdum, C. 2019. Quality by Design - A Review. PharmaTutor. 7, 4 (Apr. 2019), 48-51. DOI:https://doi.org/10.29161/PT.v7.i4.2019.48. |
INTRODUCTION
Quality by design is A Quality System for managing a product’s lifecycle, a regulatory expectation, intended to increase process and product understanding and thereby decrease patient risk, a multifunctional exercise, Design of Experiment (DoE) and Design Space. The Principle QbD as a) Risk and knowledge based decisions b) Systematic approaches process development c) Continuous Improvement d) This leads to “capable” processes. Designing for quality and innovation is one of the three universal processes of the Juran Trilogy, in which Juran describes what is required to achieve breakthroughs in new products, services, and processes[Juran, 1992]. While Quality by Design principles have been used to advance product and process quality in industry, and particularly the automotive industry, they have also been adopted by the U.S. Food and Drug Administration (FDA) for the discovery, development, and manufacture of drugs[Lawrence, 2008], [Rathore, 2009][Chemolab Lebrun, 2008][Schweitzer, 2010] .
Advantages of QbD
Benefits for Industry:
• Better understanding of the process.
• Less batch failure.
• More efficient and effective control of change.
• Return on investment / cost savings.
Understanding QbD
• Initial Confusion
• Research and discussion
• Reaching understanding
QbD development process include
• Begin with a target product profile that describes the use, safety and efficacy of the product
• Define a target product quality profile that will be used by formulators and process engineers as a quantitative surrogate for aspects of clinical safety and efficacy during product development
• Gather relevant prior knowledge about the drug substance, potential excipients and process operations into a knowledge space. Use risk assessment to prioritize knowledge gaps for further investigation
• Design a formulation and identify the critical material (quality) attributes of the final product that must be controlled to meet the target product quality profile.
• Design a manufacturing process to produce a final product having these critical materials attributes.
• Identify the critical process parameters and input (raw) material attributes that must be controlled to achieve these critical material attributes of the final product. Use risk assessment to prioritize process parameters and material attributes for experimental verification. Combine prior knowledge with experiments to establish a design space or other representation of process understanding.
• Establish a control strategy for the entire process that may include input material controls, process controls and monitors, design spaces around individual or multiple unit operations, and/or final product tests. The control strategy should encompass expected changes in scale and can be guided by a risk assessment.
• Continually monitor and update the process to assure consistent quality.
Traditional approach & Enhanced QbD approach
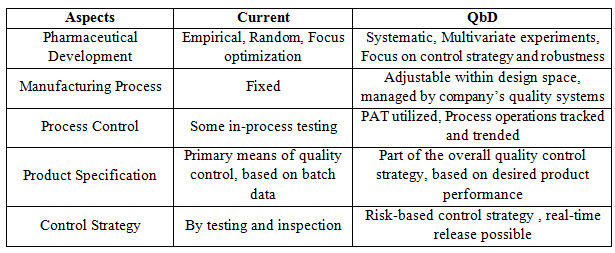
Table no .1 Traditional approach & Enhanced QbD approach
Manufacturing changes within the approved design space without further regulatory review.
• Reduction of post-approval submissions.
• Better innovation due to the ability to improve processes without resubmission to the FDA when remaining in the Design Space.
• More efficient technology transfer to manufacturing.
• Greater regulator confidence of robust products.
• Risk-based approach and identification.
• Innovative process validation approaches.
• Less intense regulatory oversight and less post-approval submissions.
• For the consumer, greater drug consistency.
• More drug availability and less recall.
• Improved yields, lower cost, less investigations, reduced testing, etc.
• Time to market reductions: from 12 to 6 years realized by amongst others.
• First time right: lean assets management.
• Continuous improvement over the total product life cycle (i.e. controlled, patient guided variability).
• Absence of design freeze (no variation issues).
• Less validation burden.
• Real time controls (less batch controls).
• Realistic risk perceptions.
• Contributes substantially to realize the better, cheaper and safer mandate.
QbD activities within FDA
Specifically, the following activities are guiding the overall implementation of QbD:
• In FDA’s Office of New Drug Quality Assessment (ONDQA), a new risk-based pharmaceutical quality assessment system (PQAS) was established based on the application of product and process understanding.
• Implementation of a pilot program to allow manufacturers in the pharmaceutical industry to submit information for a new drug application demonstrating use of QbD principles, product knowledge, and process understanding. In 2006, Merck & Co.’s Januvia became the first product approved based upon such an application.
• Implementation of a Question-based Review (QbR) Process has occurred in CDER’s Office of Generic Drugs.
• CDER’s Office of Compliance has played an active role in complementing the QbD initiative by optimizing pre-approval inspectional processes to evaluate commercial process feasibility and determining if a state of process control is maintained throughout the lifecycle, in accord with the ICH Q10 lifecycle Quality System.
• Implementation of QbD for a Biologic License Application (BLA) is progressing.
While QbD will provide better design predictions, there is also a strong recognition that industrial scale-up and commercial manufacturing experience provides new and very important knowledge about the process and the raw materials used therein. FDA is aware that knowledge is not static and builds throughout the manufacturing lifecycle. FDA’s release of the Process Validation guidance in January 2011 notes the need for companies to continue benefiting from knowledge gained, and continually improve throughout the process lifecycle by making adaptations to assure root causes of manufacturing problems are quickly corrected. This vigilant and nimble approach is explained by FDA to be essential to best protect the consumer (patient).
Integrated planning
Integrated planning requires a team with a leader whose sole accountability is for the total success of the new product from defining the opportunity through customer purchase, use, service, and recommendation to others. This team leader reports directly to a senior executive, or the team leader can be a senior executive. Each team member's job is to ensure the success of the new product [Early, 2013 part 1].
• In addition to organizational integration, a successful team must begin with clearly articulated common goals for the product that are measurable and authorized by the enterprise. These goals must, at a minimum, cover such elements as:
• The customers or customer segments to be served by the new product
• The relative and absolute quality goals
• The volume of sales or revenue to be generated in an initial time period and for the long run
• Market share, penetration, or sales relative to key competitors
• The release date
The team will follow a structured process. The structure is the common framework for all participants in launching the new product and helps ensure success.
Control over variation and transfer to operations
Quality by design incorporates modern tools to preemptively control variation. These tools and methods begin by measuring and understanding the variation that exists by using historical data, testing, and modeling to help forecast, analyze, and eliminate the deleterious effects of variation using standard statistical techniques [Early, 2013 part 2]. Process control consists of three basic activities:
1. Evaluate the actual performance of the process
2. Compare actual performance with goals
3. Take action on the difference [Joseph A, 2010]
The final activity of the quality by design process is to implement the plan and validate that the transfer has occurred.
Pharmaceutical Quality by Design
The FDA imperative is outlined in its report "Pharmaceutical Quality for the 21st Century: A Risk-Based Approach."(Pharmaceutical Quality for the 21st Century, 2007) In the past few years, the agency has implemented the concepts of QbD into its pre-market processes. The focus of this concept is that quality should be built into a product with an understanding of the product and process by which it is developed and manufactured along with a knowledge of the risks involved in manufacturing the product and how best to mitigate those risks. This is a successor to the "quality by QC" (or "quality after design") approach that the companies have taken up until the 1990s (Process Validation: General Principles and Practices , 2011).
The QbD initiative, which originated from the Office of Biotechnology Products (OBP), attempts to provide guidance on pharmaceutical development to facilitate design of products and processes that, maximizes the product's efficacy and safety profile while enhancing product manufacturability.
ICH activities
ICH guidelines Q8 (on Pharmaceutical Development), Q9 (on Quality Risk Management), and Q10 (on Pharmaceutical Quality System) provide some assistance for manufacturers to implement Quality by Design into their own operations (ICH Quality Guidelines), (CMC), (International Conference on Harmonisation). The ICH Steering Committee meets twice a year to discuss the progress of its efforts. This practical input should help ensure that quality risk management and knowledge management are used to make lifecycle adaptations that maintain process control and product quality.
CONCLUSION
Quality by Design is intended to enhance process knowledge and is based on existing guidance and reference documents. QbD is a quality system that builds on past and sets future regulatory expectations; the QbD can be viewed as a process defined by series of document requirements. These documents organize and demonstrate process knowledge and understanding. QbD can be applied to legacy and new products, but the supporting document package may differ. The QbD suite of documents is “alive”. They can and should be revised as the knowledge base changes. Ensures robust commercial manufacturing methods for consistent production of quality drugs. Ensures the consumers that therapeutic equivalent generics are manufactured every single time. QbD methodology helps in identifying and justifying target product profiles, product and process understanding. There is a need for vigorous and well funded research programs to develop new pharmaceutical manufacturing platforms.
REFERENCES
1. "Chemistry/ Manufacturing/ Controls (CMC)". Pharmatech Associates. available at: https://pharmatechassociates.com/ecosystem/productprocess/chemistry-manufacturing-controls-cmc-2/
2. "International Conference on Harmonisation (ICH) Guidelines". Rowan University. Available at: https://research.rowan.edu/officeofresearch/compliance/irb/policiesguidance/ichguidelines/index.html.
3. Chemolab Lebrun (2008); Development of a new predictive modeling technique to find with confidence equivalence zone and design space of chromatographic analytical methods; Chemometrics and Intelligent Laboratory Systems; 91(1); 4-16.
4. Department of Health and Human Services U.S. Food and Drug Administration (2007); Pharmaceutical Quality for the 21st Century: A Risk-Based Approach. Available at: https://www.fda.gov/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cder/ucm128080.htm.
5. Early, John (2013),"Quality by Design, available at: http://www.qualitydigest.com/inside/quality-insider-article/quality-design-part-1.html. (Retrieved on 03/14/2019).
6. Early, John (2013); "Quality by Design, available at: https://www.qualitydigest.com/inside/quality-insider-article/quality-design-part-2.html. (Retrieved on 03/14/2019).
7. ICH Quality Guidelines; available at: https://www.ich.org/products/guidelines/quality/article/quality-guidelines.html.
8. Joseph A. Defeo & Juran, J. M. (2010); Juran's Quality Handbook: The Complete Guide to Performance Excellence 6/e; McGraw-Hill Education; 6 edition; 1136.
9. Juran, J. M. (1992); Juran on Quality by Design: The New Steps for Planning Quality into Goods and Services Free Press Business & Economics; 538.
10. Lawrence X. Yu (2008); Pharmaceutical quality by design: product and process development, understanding, and control; Pharm Res; 25(4); 781–791.
11. Rathore A.S. (2009); Roadmap for implementation of quality by design (QbD) for biotechnology products; Trends Bio technol.; 27(9):546-53.
12. Schweitzer, Mark. (2010); "Implications and Opportunities of Applying QbD Principles to Analytical Measurements"; Pharmaceutical Technology; 34(2); 52–59.
13. U.S. Department of Health and Human Services Food and Drug Administration (2011); Process Validation: General Principles and Practices" (PDF). FDA Guidance. Available at: https://www.fda.gov/downloads/drugs/guidances/ucm070336.pdf.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









