 About Authors:
About Authors:
Sanober Parveen*, Maju Vyas Singh
Delhi institute of Pharmaceutical Sciences & research University (DIPSAR), Delhi.
*sanober.parveen@gmail.com
Abstract
The present study was aimed Pharmacognostic and preliminary phytochemical study of the fresh fruits of Alstonia scholaris, belonging to family Apocynaceae.The pharmacognostic investigation were carried out in terms of macroscopic, microscopic and physical parameters. The extract obtained after successive Soxhlet extraction of dried fruit using n-hexane, chloroform, methanol and water were subjected to a preliminary phytochemical screening which revealed the presence of alkaloids, carbohydrate, glycoside, terpenoids and flavanoids.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1452
Introduction
Alstonia scholaris which is popularly known as the “Saptaparni” or “Devil’s tree” belonging to the family Apocynaceae. It is widely distributed in dried forests of India, western Himalayas and in the Southern region. The whole plant is medicinally useful. It is a well known remedy for the treatment of various types of disorders in the Ayurvedic, homeopathic and folklore system of medicine in india. The plant is a constituent of Ayurvedic preparation Ayush-64 which is found effective in microfilaraemia.(1)
Previous investigation revealed that the various part (leaves, bark, flowers) of this plant Alstonia scholaris have antibacterial activity, anti-malarial, anti-amoebic, anti-plasmodial, anxiolytic activity, antiinflamatory, analgesic, anti-ulcerogenic activity, anti-fertility, anticancer activity. Analysis of phytochemical constituents (Chakravarti et al., 1955, 1956; Talapatra et al., 1967, 1968; Banerji and Banerji, 1975; Dhar et al., 1977; Banerji and Chakrabarti, 1984; Banerjee et al., 1984; Arambewela and Ratnayake, 1991; Varshney and Goyal, 1995; Mahajan and Badgujar, 2008; Deepthi et al., 2008; Khyade and Vaikos, 2009a; Thenmozhi et al., 2010; Dutta et al., 2010;
[adsense:468x15:2204050025]
Thankamani, 2011) and pharmacognosy (Datta and Datta, 1984; Upadhye et al., 2006; Ansari et al., 2006; Hemalatha et al., 2008; Dutta and Laskar, 2009; Khyade and Vaikos, 2009b) of the species have been reported by many authors. Alkaloids are one of the major constituents of the speciesEchitamine chloride (Kamarajan et al., 1991; Saraswathi et al., 1997,1998,1999); Rhazine (Chatterjee et al., 1969); Nareline (Morita et al., 1977); Pseudo Akuammigine (Banerji and Banerje, 1977); Scholarine (Banerji and Siddhanta, 1981); Scholaricine (Banerji, 1981; Rahman et al., 1985); Dihydrocondylocarpine, (Rahman et al., 1986); 19, 20-Z-Vallesamine and 19, 20-EVallesamine (Rahman et al., 1987); Picrinine (Ghosh et al., 1988); Alschomine and Isoalschomine, (Abe et al., 1989); Mataranine A and B (Hadi, 2009); monoterpenoid indole alkaloids (Cai et al., 2007; 2008a; Feng et al., 2009); Picralinal of picralima group (Rastogi et al., 1970); Picrinine-type alkaloids (Cai et al., 2008b); N1-methoxymethyl Picrinine (Wang et al., 2009) etc. have been reported. Constituents have been reported from different parts of the plant such as bark (Manohar and Ramaseshan, 1961; Yamauchi et al., 1990b; Gupta et al., 2002; Salim et al., 2004; Feng et al., 2009); leaves (Chatterjee et al., 1965; Banerji and Banerje, 1977; Rahman et al., 1986; Yamauchi et al., 1990a,b; Zhou et al., 2005; Macabeo et al., 2005; Cai et al., 2008b; Hirasawa et al., 2009); roots (Boonchuay and Court, 1976b); flowers (Dutta et al., 1976) and fruits (Wongseripipatana et al., 2004) Among the other constituents, Isookanin-7-o-alpha-lrhamnopyranoside, a new flavanone glycoside (Chauhan et al., 1985) and Alstonoside, a secoiridoid glucoside (Thomas et al., 2008) have been recorded. Iridoids, coumarins, flavonoids, leucoanthocyanins, reducing sugars, simple phenolics, steroids, saponins and tannins were also found in the plant (Khyade and Vaikos, 2009a).
However, very little is understood about the Pharmacognostic study of the fruit of alstonia scholaris. Taking the same in view, attempts have been made toPharmacognostic and Phytochemical study of the plant.
Material and Method
Collection of plant material
The fresh Fruits of Alstonia scholaris (L.) R. Br. were collected from the campus of DIPSAR, Pushp Vihar, sec-3, India, in the month of March 2011. The Plant sample was authenticated and Voucher specimen has been deposited at the NISCAIR, new Delhi-12, with the reference No. NISCAIR/RHMD/Consult/-2011-12/1732/32.
Pharmacognostic studies
1. Macroscopic study
Macroscopic characters shape, size, color, odor, texture etc. of the plant material were studied.
2. Microscopic study
a. Preparation of sections
A piece of the plant material was selected. Thin and fine transverse sections were prepared by cutting with razor blade. Satisfactory sections were selected, cleared by using a solution of chloral hydrate and stained with Phoroglucinol and conc. HCl. Stained section was mounted in glycerine and examined under the microscope.
b. Powder Microscopy
A small amount of powder was cleared using chloral hydrate solution and stained with phloroglucinol and conc. HCl. It was mounted in glycerine and examined under microscope.
3. Fluorescence Analysis
The powdered drug was treated with different reagents ( 1N HCl, 1N NaOH, 50% H2SO4, 50% HNO3 ), and examined under Day light and UV-light (at 254nm & 366nm). The results were recorded in Table 2.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
PHYSICAL PARAMETERS
1. Ash Value
The ash of any organic material is composed of their non-volatile inorganic components. Controlled incineration of crude drug results in an ash residue consisting of any organic material (metallic salts and silica). More direct contamination, such as by sand or soil, is immediately detected by the ash value.
a. Determination of Total Ash Content
About 3g of powdered drug was taken in silica dish and incinerated in muffle furnace at a temperature not exceeding 450oC until it became carbon free, then allowed to cool & weighed. The percentage of total ash was calculated with reference to air dried drug.
b. Determination of Acid Insoluble Ash Content
The ash obtained from the drug was boiled with 25ml of dilute hydrochloric acid for 5 minutes. The insoluble matter was collected on Gooch crucible, washed with hot water and ignited to dull redness, allowed to cool & weighed. The percentage of acid insoluble ash was calculated.
c. Determination of Water soluble Ash Content
The total ash obtained from the drug was boiled with 25ml of water for 5 minutes. The insoluble matter was collected on Gooch crucible, washed with hot water and ignited for 15 minutes at a temperature not exceeding 450oC. Substract the weight of insoluble matter from the weight of the Ash; the difference in weight represents the water soluble ash.(Ayurvedic Pharmacopoeia, Part I, vol. I )
2. Loss on drying
The test for loss on drying determines both water and volatile matter. Drying was carried out by heating to 100°C-105°C in an oven.
Method
About 5g of the air-dried powderd drug was taken and accurately weighed the petri dish with contents. The sample was dried in an oven at a Temp. 1050C, until constant weight of sample was obtained. After drying was completed, the petri dish was allowed to cool at room temperature in a Desiccator. The loss of weight in mg per g of air-dried material was calculated.
3. Extractive value
Extractive value determines the amount of active constituents in a given amount of medicinal plant material when extracted with solvents.
a. Determination of Alcohol Soluble Extractive value
About 5g air?dried, coarsely powdered material was placed in a glass stoppered conical flask and macerated with 100 ml of Alcohol (90% v/v) for 6 hours, with frequent shaking and then allowed to stand for 18 hours. It was then rapidly filtered through Whatmann filter paper, to prevent loss of any solvent. 25 ml of filtrate was transferred to flat?bottom dish and solvent was evaporated on a water bath, then dried at 105 °C for 6 hours and kept in a desiccator for 30 minutes and weighed.
b. Determination of Water Soluble Extractive Value
About 5g air?dried, coarsely powdered material was placed in a glass stoppered conical flask and macerated with 100 ml of Chloroform:water (0.25:100) for 6 hours, with frequent shaking and then allowed to stand for 18 hours. It was then rapidly filtered through Whatmann filter paper, to prevent loss of any solvent. 25 ml of filtrate was transferred to flat?bottom dish and solvent was evaporated on a water bath, then dried at 105 °C for 6 hours and kept in a desiccator for 30 minutes and weighed .
Preliminary phytochemical studies
The preliminary phytochemical study was carried out to check the presence of phytoconstituents in different extracts (acetone, chloroform, methanol, water) of Alstonia scholaris fruit. The qualitative test gives the general idea regarding the nature of chemical constituents of the crude drugs. The extract was subjected to Preliminary phytochemical investigation for detection of:
1. Alkaloids
Dragendorff’s Test:The plant extract was mixed with a few drops of acetic acid followed by Dragendorff’s reagent and mixed well. A orange red precipitate is formed indicated the presence of alkaloid.
Wagner’s Test:The crude extract mixed with wagner’s reagent. Reddish brown precipitate indicate the presence of alkaloids
Hager’s Test:The crude extract mixed with Hager’s reagent. Yellow color precipitate indicate the presence of alkaloids.
2. Glycosides
About 5 ml of the plant extract was treated with 2 ml of glacial acetic acid containing a drop of ferric chloride solution. Then it was underplayed with 1 ml conc. H2SO4. A brown ring of the interface indicates the presence of glycosides.
Keller killiani test: Plant material was treated with chloroform. Add 0.4ml of glacial acetic acid containing a traceamount of ferric chloride. Then add carefully 0.5ml of conc. H2SO4 by theside of the test tube, blue color appears in the acetic acid layer, indicates the presence of cardiac glycosides.
Modified Borntrager’s test: About 2ml of plant extract was mixed with 2 ml of dil. H2SO4 and 2 ml of 5% aqueous ferric chloride sol & boiled for 5 minutes. Filtered, cooled and shake with an equal volume of chloroform, seperated the lower layer of chloroform and shake with half its volume with dilute ammonia. A rose pink to red color is produced in the ammonical layer, indicates the presence of anthraquinone glycosides.
3. Steriods
Libermann-buchard test:Crude extract mixed with few drops of acetic anhydride, boiled and cool, conc. H2SO4 was then added to sides of the test tube. A brown ring at the junction of two layers was formed. The upper layer turned green which shows the presence of steroids.
4. Flavonoids .
Shinoda Test:Crude extract was mixed with few fragments of magnesium ribbon and conc. HCl was added dropwise from the sides of test tube. Red color appeared after few minutes, which indicates the presence of flavanoids.
5. Tannins and Phenolic Compound
Tannins (ferric chloride test):About 0.5 g of the dried powdered sample was boiled in 20 ml of water and then filtered. A few drops of 0.1% ferric chloride was added. A blue-black coloration, indicates the presence of tannin.
Phenols: A few drops of alcohol and ferric chloride solution was mixed with the plant extract. A blue green or red color indicates the presence of Phenol.
6. Terpenoids
About 5 ml of the plant extract was mixed in 2 ml of chloroform and conc. H2SO4 was added dropwise from the sides of test tube to form a layer. A reddish brown coloration of the interface was formed to show the presence of terpenoids.
Salkowski test: Crude extract was mixed with chloroform and a few drops of conc. H2SO4, shaked well and allow to stand for some time. Formation of yellow colored layer indicates the presence of triterpenoids
7. Saponin
Foam test: About 2g of the powdered sample was boiled in 20 ml of distilled water bath and filtered. The 10 ml of the filtrate was mixed with 5 ml of distilled water and shaken vigorously for a suitable persistent froth.
8. Carbohydrates (Fehling’s test)
The extracts were treated with 5.0 ml of Fehling’s solution (A+B) and kept in boiling water bath. The formation of yellow or red colour precipitate indicates the presence of reducing sugars.
9. Amino acids and Proteins
Ninhydrin test:To 1ml extract, 2 drops of freshly prepared 0.2% ninhydrin reagent was added and heated. Blue colour develops indicating the presence of amino acids and proteins.
10. Fixed oils and fats (spot test) Press a small quantity of powder between two filter papers. Oil strains on the filter paper indicate the presence of fixed oils.
Result and Discussion
Pharmacognostic study
1. Macroscopic study
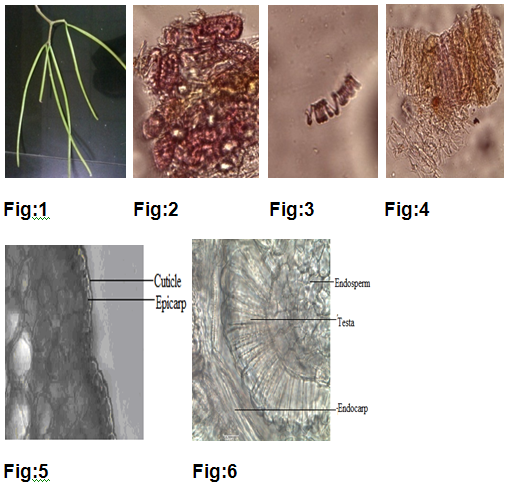
Young fruits are straight, green in color, 28-30cm long, 3-4mm in thickness, externally smooth, internally green in color with growing stage of embryo in young stage. On maturation, splits longitudinally bears brown color seeds, possessing brown hairs.(fig1)
2. Microscopic Study
a. TS of Fruits of Alstonia scholaris
Transverse section of fruit shows Pericarp, testa and endosperm.
Pericarp (fruit covering) consists of a single layer of polygonal cells of epicarp covered with thick cuticle.Multilayered mesocarp made up of parenchymatous cells showing vascular bundles and orange colored latex cells. Double layer of Endocarp.Testais made up of elongated cells and endosperm made up of polygonal parenchymatous cells which contains orange colored Latex cells.(fig5,6,7).
b. Powder Microscopy
Brown colored powder of drug under microscope showed in fig2,3,4 & table1.
3. Phytochemical analysis
Preliminary phytochemical analysis of the extracts gives the general idea of the type of chemical constituent present in crude drug sample. The result showed the presence of alkaloids, glycosides, tannins in all extracts. The aqueous extract and methanolic extract of plant sample showed presence of maximum number of phytoconstituent. Only chloroform extract showed the presence of terpenoids.(Table 4)
Tables & Figures
Table:1
|
S.No. |
Observation of powder |
|
1 |
Sclereids without lumen |
|
2 |
Spiral Vessels |
|
3 |
Orange colored latex cells |
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Table:2 Fluorescence analysis
|
Chemical Treatment |
Day Light |
UV-Light |
|
|
Short wavelength |
Long wavelength |
||
|
Powder |
Light brown |
Light brown |
Light Brown |
|
1N HCl |
Light brown |
Light brown |
Brown |
|
1N NaOH |
Brown |
Green |
Brownish green |
|
50% H2SO4 |
Light brown |
Light brown |
Brown |
|
50% HNO3 |
Light brown |
Light brown |
Brown |
Table:3
|
S.no. |
Standardization parameters |
Results |
|
1 |
Total ash |
8.3 |
|
2 |
Acid insoluble ash |
2.3 |
|
3 |
Water soluble ash |
2 |
|
4 |
Loss on drying |
7 |
|
5 |
Alcohol soluble extractive |
4.8 |
|
6 |
Water soluble extractive |
12.6 |
Table:4 Phytochemical analysis
|
Phytochemical compound |
Chloroform extract |
Acetone extract |
Methanol extract |
Aqueous extract |
|
Carbohydrates |
- |
+ |
- |
+ |
|
Glycosides |
+ |
+ |
+ |
+ |
|
Saponins |
- |
- |
+ |
+ |
|
Fixed oils |
- |
- |
- |
- |
|
Terpenoids |
+ |
- |
- |
- |
|
Alkaloids |
+ |
+ |
+ |
+ |
|
Steroids |
- |
- |
- |
- |
|
Flavanoids |
- |
- |
+ |
+ |
|
Tannins |
+ |
+ |
+ |
+ |
|
Amino acids and Proteins |
- |
- |
+ |
+ |
( + Present, - Absent)
Acknowledgement
The authors are thankful to Dr. B.P. Srinivasan, Director,Delhi institute of Pharmaceutical Sciences 7 research University (DIPSAR), Delhi.
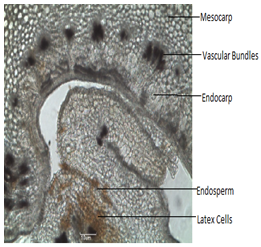
Fig:7
References
* Agrawal SS, Paridhavi M.;Herbal drug Technology; 1st edition; 2007; pg nNo.1-3 & pg nNo. 625.
* Ayurvedic Pharmacopoeia of India (API), Govt. of India, Ministry of Health and Family Welfare, Department of Indian System of Medicine and Homoeopathy, New Delhi, I- Edition, Part-I, Vol. I : 1999 ; pg 97-98..
* Pullok K. Mukherjee; Quality Control of Herbal Drugs; I- Edition (2002); pg No 186-219 & pg No. 428,441,448.
* Quality standards of Medicinal Plants; ICMR;2005, Vol. 3; pg no. 49-54.
* Review on Indian medicinal Plants; Vol. 2 (Alli-Ard); pg no. 132-137.
* Wealth of India-(Raw Materials); Vol. I : A, pg no. 201-204.
* Wealth of India; first supplement series (Raw Materials); Vol. I : (A-Ci).
* Ck Kokate; Practical Pharmacognosy; 4th edition (1994); pg No. 7-9.
* S.Wongseripipatana et al; Indole Alkaloids from the fruits of Alstonia scholaris; Thia J. Pharm. Sci. 28 (3-4): 173-180 (2004).
* Thenmozhi. M et al; A comparative Phytochemical analysis of the Leaves of Alstonia scholaris, Lawsonia inermis, Ervatamia divaricata and asparagus racemosus; International Journal of Pharma. Research and Development (IJPRD); issue 9, 13 Nov. 2010.
* Vaidyanath Iyer Thankamani et al; Anti-microbial activity of alstonia scholaris Flowers; International Journal of Pharma. Research and Development (IJPRD); Vol. 3(4): pp. 172-178; (2011).
* Khyade, M. S. and Vaikos N. P. ; Phytochemical Screening and Antibacterial activity of Leaves of Alstonia scholaris; African journal of Biotechnology, Vol. 8 (22), pp. 6434-6436,(2009).
* Kuntal Das, Raman Dang, Manjunath U. Machale; ); Formulation and Evaluation of Herbal Gel of stevia extract Iranian journal of Dermatology,Vol 12,No. 4;(2010); pg No. 117-122.
* Mohini Phanse, Parag Kulkarni, shailesh Kewatkar, Meghna Lande, Santosh Bhujbal, Pravin Chaudhri; Evaluation of anti inflammatory activity of Herbal gel formulation Scholars Research Library; Journal of Natural Product Plant Resources; (2011);;pg No. 25-28.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









