 About Authors:
About Authors:
Venkata Naveen Kasagana*, Swathi sree Karumuri
Department of Pharmaceutics
Sankaralingam Bhuvaneswari College of Pharmacy, Anaikuttam,
Sivakasi - 626 130, Tamilnadu, India.
*naveen.kasagana@gmail.com
Abstract:
In the recent past global pharmaceutical markets has involved in developing the new Novel and Advanced drug delivery systems in a safe & cost effective manner. This resulted in the introduction of a variety of Controlled and Targeted drug delivery methods with ease of administration and patient compliance. The transition that took place by the introduction of nano technology in the field of pharmacy leads to the development of many intelligent drug delivery systems like Nano Beacon and Mini Beacons, Trojan horse Nano particles, MEMS & NEMS (Micro and Nano Electromechanical Systems). Cochleate delivery vehicles “A novel lipid-based system” represent a unique technology platform for oral and systemic delivery of drugs. BEMA (Bio Erodible Muco Adhesive Disc) drug delivery technology for prolonged acting action has contribution to the novel approach. Also a much awaited Non-injected insulin formulation “Nasal Insulin spray”, as a vaccine and also in the treatment of Type-II diabetes, provided a shift in the development of drug delivery systems. Duocap (Capsule-in-a-capsule), Electro capsule, Light up delivery monitoring technology (LDMT) are some of the controlled & targeted oral drug delivery systems, which provides a new way of formulating poorly soluble compounds and monitor the release rate of the drug. Apart from briefly describing the above technologies, the article includes the upcoming system of using Air as a barrier for Controlled release and application of medical devices as drug and their contribution to the World of pharmacy.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1446
Introduction:
Pharmaceutical and biopharmaceutical firms are in search of drug delivery systems that can meet the requirements of patients and also reduce the cost and lead time involved drug development. A range of recently developed technologies makes that goal possible. Once-a-day formulations are a holy grail of sorts for scientists working with oral dosage forms. This ideal dosing regimen, which enhances patient compliance and helps guard against overdosing and side effects, is made possible by controlled-release delivery systems, which use a variety of mechanisms to deliver and maintain the drug at a certain level in the patient's bloodstream. This can be achieved by the introduction Nano & Novel drug delivery technologies.
[adsense:468x15:2204050025]
Nano Beacon:
The past few years have seen major advances in the design of ‘molecular vehicles’ – particles/Capsules that can be filled with a medicine or new genes. One of the major roadblocks that have encountered in designing these molecular transport systems is how to get the vehicle contents out of their container once they reach the site where they are needed.Nano-Beacon technique overcomes this problem and allowing the scientists to rapidly and accurately determine the permeability of DNA through Membrane pores.1 The beacons are single DNA strands which have a light-emitting molecule (a fluorophore) at one end and a quencher at the other.The linear molecule contains a palindrome DNA sequence that loops on itself and undergoes complementary base pairing, opposing the fluorophore and its quencher together at the narrow end of the flask-shaped molecule. When the 'nano-beacons' are placed inside the capsules, they are in their base-paired conformation, and emit no fluorescence. Single-stranded DNA 'probes' of various lengths, but complementary to the DNA sequence of the beacon, are introduced into the medium containing the capsules. If they are of the right size to enter the microcapsule through its membrane pores, they hybridise competitively with one or other of the base-paired sequences of the 'flask'. As they do so, they forcing the strands apart, separating the fluorophore from its quencher -- and the fluorophore emits light, with intensity proportional to the number of 'probe' molecules that penetrate the microcapsule. The slow increase in light output allows the drug developer to calculate how rapidly the DNA is penetrating.2 These mini-sized biomarkers/beacons are used to seek out certain disease-indicating genes and glow when they find them. This created new methods for early disease detection, imaging and drug delivery.3, 4
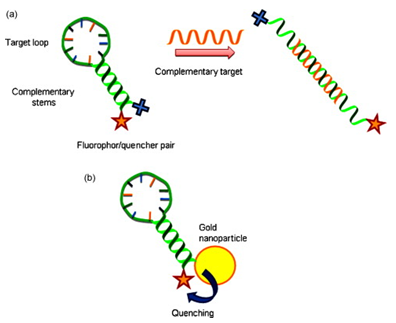
Trojan horse Nano particles:
The development of BBB drug targeting technology is an arcane area of discovery medicine that suffers from chronic under-development. Although it is generally regarded that small molecule drugs cross the BBB, in fact, >98% of all small molecule drugs do not cross the BBB (Pardridge, 2001). Essentially 100% of large molecule drugs do not cross the BBB. A new solution to the brain drug delivery problem is the genetic engineering of recombinant fusion proteins.5 The therapeutic peptide or protein drug is fused to a molecular Trojan horse, which is a second peptide that binds a specific receptor on the BBB. The Trojan horse enables receptor-mediated delivery of the fusion protein across the BBB so that the protein drug can enter the brain and exert the desired pharmacological effect.
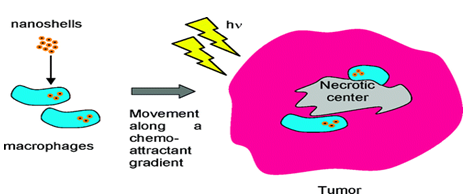
Also one of the most difficult feats to accomplish with today’s anticancer therapies is getting drug into the oxygen- and nutrient-deprived cores of solid tumours. These inaccessible regions may be the source of drug-resistant tumours. This was overcome by the use of nanoparticle-loaded Trojan horses which can penetrate the tumour mass. Once inside the tumour core, the nanoshells can be activated using near-infrared light, turning them into miniature thermal scalpels that kill tumours from the inside out.6,7
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Micro & Nano electro mechanical Drug Delivery Systems 8
Microelectro mechanical system (MEMS) & Nano Electromechanical Systems(NEMS) devices offer minimally invasive and allow control of the delivery site and amount. For example, some drugs, such as hormones, may be more effective when released in a manner similar to the way it would be produced naturally. Through the use of these technologies and micro fabrication techniques, drug delivery research has been able to make a significant departure from traditional methods. Research has branched MEMS and NEMS into different areas. Some of them are In vivo devices, transdermal devices, Reservoir Implantation Device.
In Vivo Devices9,10
These MEMS devices can be positioned in the body by implantation or by the traditional pill. One promising such device is a chip that contains micro reservoirs full of the prescribed drug.
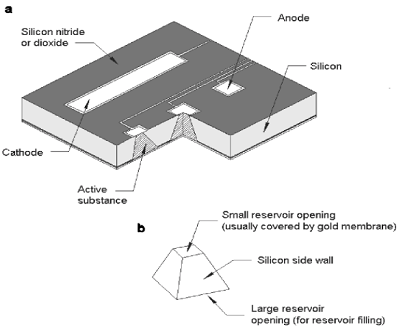
The reservoirs are created on the substrate using micro fabrication techniques and are then filled with the drug. The drugs contained in the reservoirs are released by a variety of different techniques. However, the small size also means a great deal of these reservoirs can be placed on a single device suggesting one device could last for very long periods of time. Also, different reservoirs can be filled with different drugs; so one device could contain all the drugs a person requires.
Similar to the self-administering device mentioned above is the so-called smart pill. This matchstick sized device can be implanted into the body of a patient.10,11
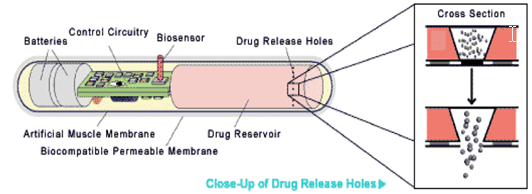
Equipped with an external sensor, the smart pill detects the conditions in its surrounding environment. This information is passed on to a chip where the information is processed. The chip then determines the course of action to take. A signal is sent to the included battery pack, which actuates a membrane. The membrane contracts and just the right amount of the drug is released.
A. Transdermal Devices12
As opposed to In vivo devices, transdermal devices deliver the drug through the skin. Micro needles created by micro fabrication techniques are used to improve the effectiveness of transdermal drug delivery.
Micro needles
- Micro needles were first developed as a method for transdermal drug delivery. The microneedles are long enough to penetrate the stratum corneum, which often acts as the primary barrier to drug transport across the skin, but are short enough to avoid the nerves located in the dermis, potentially offering a painless method for drug delivery.
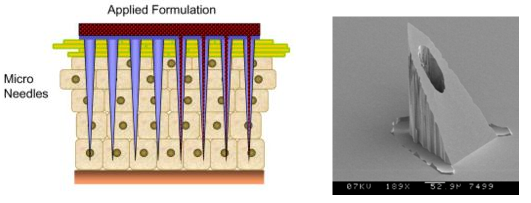
SEM Picture of a 350 micron high micro needle, with a base of 250 microns, the flow channel is 70 microns in its widest direction.
The needles are fabricated with channels through them. The drug is then pumped through the channels into the body. Since the channels are so small, the effects on the skin are insignificant.
B. Reservoir Implantation Device[13]
Of the different NEMS devices, the implantation devices were found to be the best choice based on the fact that they offer a greater deal of control and are more effective than the transdermal techniques. The reservoir implantation device was chosen over the smart pill. Because the device can be activated by remote control, giving control to the doctor or the patient. It can be activated on a set time basis.

Some devices are even automatically triggered by sensors built into the device that detect when the drug needs to be administered.
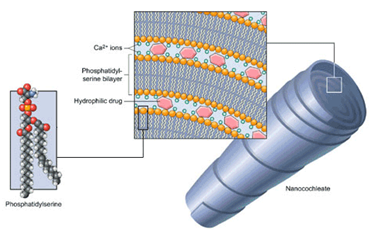
Cochleate drug delivery14,15
Cochleate delivery vehicles represent a new technology platform for oral and systemic delivery with important therapeutic biological activities, including drugs, genes, and vaccine antigens. Cochleate formulation technology is particularly applicable to macromolecules as well as small molecule drugs that are hydrophobic, positively charged, negatively charged, and that possess poor oral bioavailability. Cochleate delivery vehicles are stable phospholipid-cation precipitates composed of simple, naturally occurring materials (ie, phosphatidylserine and calcium). They have a unique multilayered structure consisting of alternating layers of calcium and phospholipid in large, continuous, solid, lipid bilayer sheets rolled up in a spiral or stacked, with little or no internal aqueous space. This structure provides protection from degradation for associated "encochleated" molecules. Calcium-induced perturbations of membranes containing negatively charged lipids and the subsequent membrane fusion events are important mechanisms of this type of drug delivery. Therefore, cochleates can be envisioned as membrane fusion intermediates. The calcium-rich, highly ordered membrane of a cochleate first comes into close approximation to a natural membrane, a perturbation and reordering of the cell membrane is induced, resulting in a fusion event between the outer layer of the cochleate and the cell membrane. This fusion results in the delivery of a small amount of the encochleated material into the cytoplasm of the target cell. The cochleate could then break free of the cell and be available for another fusion event, either with this or another cell. Alternatively, particularly with active phagocytic cells, the cochleate may be taken up by endocytosis and fuse from within the endocytic vesicle. Cochleates made with trace amounts of fluorescent lipids have been shown to bind and gradually transfer lipids to the plasma membrane and interior membranes of white blood cells. Using Amphotericin B (AmB) as a model, cochleates have been shown to be highly effective at mediating the oral delivery of drugs that are currently only available in injectable formulations. Cochleates for the Delivery of OTC Anti-inflammatory Agents including aspirin, ibuprofen, naproxen, acetaminophen, and COX-2 inhibitors. Cochleates represent a powerful subunit vaccine delivery system, uniquely suited to meeting the challenges of modern vaccine development. Other drugs with cochleate technology are Doxorubicin, Cyclosporin A, Nelfinavir, Rifampin, Vitamin-A and even DNA loaded cochleates are available.
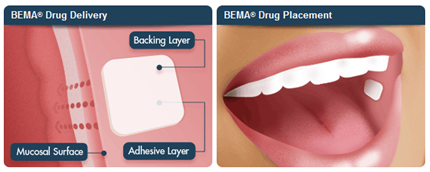
BEMA Technology16
The BEMA drug delivery technology consists of a small, bio erodible polymer film for application to the mucosal membranes (inner lining of cheek). BEMA films were designed to rapidly deliver a dose of drug across the mucous membranes for time sensitive conditions or to facilitate administration of drugs with poor oral (pill) absorption. BEMA films are designed to:
- Adhere to oral mucosa in less than 5 seconds
- Optimize delivery across the oral mucosa
- Completely dissolve within 15 to 30 minutes
Onsolis (fentanyl buccal soluble film) is the first product to be developed and marketed using the BEMA technology. The BEMA technology may also be developed with other active drugs, particularly in conditions where rapid delivery of drug is important, oral dosing is not optimal, or where intravenous line or injections are unavailable or not practical.
Bio Delivery Sciences International (BDSI) has acquired the rights to the BEMAoral drug delivery technology.
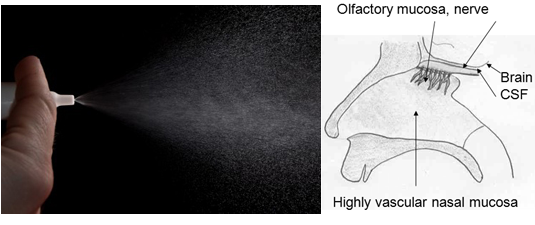
Nasal Insulin spray17
A new study released Monday shows that insulin applied daily through a special nasal spray might be a treatment that slows or stops the progression of Alzheimer’s disease. Alzheimer's disease, the most common form of dementia in the elderly, initially causes difficulty with thought, memory and language, and insulin dysfunction.In recent years, many studies in humans and animals have found that the insulin resistance which is the hallmark of type 2 diabetes plays a critical role in the development of Alzheimer’s. Simply giving a person, insulin with the usual method used to treat diabetes would not solve the problem. In fact, it could be very dangerous. Nasal Insulin spray,a special device that inserts the insulin into the sinus cavities closest to the brain. From there, the protein travels along nerve cells reaching the brain within 15 to 20 minutes.
DuoCap – “A capsule in Capsule”18
Over recent years, controlled release combination products have become increasingly popular within the pharmaceutical industry. Controlled release combination products present complex challenges to formulators and manufacturers such as physico-chemical and biological compatibility, process scale-up and packing. An ideal solution is DuoCap delivery system.
DuoCap Delivery System has
a. Two independent compartments in one dosage unit.
b. Inner capsule contains liquid, semi-solid or powder formulation.
c. Outer capsule contains liquid or semi-solid formulation.
d. Drug delivery targeted to two different regions of GI tract.
e. Sustained, Pulsed or delayed release profiles
Advantages of DuoCap Delivery System
a. Novel formulation combinations.
b. Controlled & Multi-phase release.
c. Patient Compliance & Convenience.
d. Cost – effective therapy.
e. Single oral dosage unit.
f. Pilot to commercial manufacturers.

Electro capsule19
Intellicap – an orally administered wirelessly controlled electronic capsule system – is the first in the world to mark its position In vivo using both pH and temperature detection sensors. It focuses specifically on absorption delivery in the gut. Once in the desired location, the medication can either be released by an operator, or through the device itself which can be pre-programmed in a controlled way not available previously. This type of drug delivery can not only track but also see how release of drug takes place at the target site.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Air as Barrier for Controlled Drug Release20
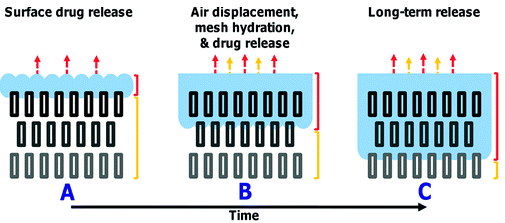
A new study has found a way to use air as a barrier for controlled drug release. This technology uses drug-loaded polymeric meshes with a removable air pocket that acts as a barricade. When the air was removed, the drug is released and the release rate could be extended for a period of two - three month. Mesh used is a porous 3D super hydrophobic electrospun material which was prepared using biocompatible building blocks in which the drug is entrapped. Water penetrates into the porous network and displaces the entrapped air. Penetration of air depends on the contact angle of mesh. Thus a controlled release rate of drug can be achieved.
Medical Devices as Drugs21
Recent reports indicate a potential shift in healthcare as researchers and doctors are increasingly just saying no to drugs and instead embracing medical devices to treat certain conditions. Mitral valve prolapse is a heart problem in which the valve that separates the upper and lower chambers of the left side of the heart does not close properly. In the past, some people with mitral valve prolapse were given antibiotics before certain surgical procedures to help prevent an infection called bacterial endocarditis. Other drugs that may be prescribed when mitral regurgitation are Water pills (diuretics) help remove excess fluid in the lungs, Propranolol for palpitations or chest pain. Sometimes surgery is required to repair or replace the valve if you have severe mitral regurgitation. The MitraClip system is a catheter-based therapy intended to reduce mitral regurgitation (MR).
References:
1. Elaine Mulcahy, Nano beacon will light way for smarter drug delivery, UniNews, Vol. 14, No. 14 8 - 22 August 2005.
2. Graeme O'Neill, Melbourne Uni's 'nano-beacon' plays tricks with DNA, Australian Life Scientist, 05 August, 2005.
3. nano.gatech.edu/faculty-staff/profiles/gangbao.php.
4. Angus Johnston, Frank Caruso, Elaine Mulcahy, Mini-beacon design a major development for smart drug delivery, Chemical and Bimolecular Engineering. Website: voyle.net/Nano Medicine 2005/Medicine 2005-0093.html.
5. William M pardridge, Molecular trojan horses for blood-brain barrier drug delivey, discovery medicine, July 27,2009.
6. William M. Pardridge, The Future of Brain Drug Development, Brain Drug Targeting, may 2001.
7. Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, Badve S, Sturgis J, Robinson JP, Bashir R, Halas NJ, Clare SE., A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors., pubmed, Dec 2007.
8. D.Emanuele, Antony, and John . N .An Electrically Modulated Drug Delivery Device: I. Pharmaceutical Research, 1991; Volume 8(7): 913-918.
9. Khademhosseini, Alireza. Controlled-release Microchip Drug Delivery Systems:Past, Present, and Future. Retrieved on November 30, 2004 from the World Wide Web: tissueeng.net/ali/papers/Drug%20Delivery.html.
10. Santini, John. Implantable MEMS Drug Delivery Devices. Microchips, Inc.Retrieved on Nov.28, 2004 from the World Wide Web: njnano.org/pasi/event/talks/cima.pdf.
11. Products. ChipRX, Inc. Retrieved on December 1, 2004 from the World Wide Web: chiprx.com/products.html.
12. Dario, Paolo, Chiara Carrozza, Maria, Benvenuto, Antonella, et al. .Micro-Systems in Biomedical Applications. Journal of Micromechanics and Micro engineering (2000) Volume 10(2), 235-244.
13. Santini, John T. BioMEMS for Drug Delivery, From Concept to Commercialization. Boston University Emerging Technologies Series. Boston,MA. May 10, 2002.
14. Dr. David Delmarre, Dr. Susan Gould-Fogerite, Dr. Raphael J. Mannino., Formulation of Hydrophobic Drugs Into Cochleate Delivery Vehicles: A Simplified Protocol & Bioral Formulation Kit, drug development & delivery, Vol. 4(1) 2004.
15. Susan Gould-Fogerite, Masoumeh Kheiri, Fan Zhang and Raphael J. Mannino., Cochleate Delivery Vehicles: Applications in Vaccine Delivery, journal of liposome research, vol. 10(4) 2000.
16. BEMA delivery technology, bio delivery sciences international. Website: bdsi.com/BEMA_Technology.aspx.
17. Robert bazell, insulin nasal spray may slow Alzheimer’s. msnbc.msn.com/id/44489974/ns/health-alzheimers_disease/t/insulin-nasal-spray-may-slow-alzheimers.
18. encapdrugdelivery.com/Encaps-drug-delivery-technologies/duocap-capsule-in-capsule-technology.
19. Natalie Marrison., electronic targeted drug delivery technology. Website: in-pharmatechnologist.com
20. Stefan T. Yohe, Yolonda L. Colson, Mark W. Grinstaff. Superhydrophobic Materials for Tunable Drug Release: Using Displacement of Air To Control Delivery Rates. Journal of the American Chemical Society, 2012.
21. Debra Sherman and Bill Berkrot., Analysis: Devices take lead in future heart disease battle, Reuters,april 2011.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









