About Author: Mr. Naresh. M. Reddy
PG Diploma (Cheminformatics),
Nizam College, Osmania University
Hyderabad.
Abstract
Oral strontium ranelate is an alternative oral treatment, belonging to a class of drugs called dual action bone agents. The objective of this investigation was to characterize and determine the effect of the Strontium ranelate on Calcium sensor receptor (CASR) which is an important protein involved Osteophoresis. Homology modeling of CASR has been performed based on the crystal structure of the 2E4U (Chain A) by using Modeller software. With the aid of the molecular mechanics and molecular dynamics methods, the final model is obtained and is further assessed by procheck and verify 3D graph programs, which showed that the final refined model is reliable. With this model, a flexible docking study of CASR with a group of Ranelic acids were selected from the previous publications was performed. The results indicated that VAL1,SER18,SER2,GLU241 in CASR are important determinant residues in binding as they have strong hydrogen bonding with Ranelic acids. These hydrogen binding interactions play an important role for stability of the complex. Among the 15 compunds, 6th showed best docking result. Our results may be helpful for further experimental investigations.
[adsense:336x280:8701650588]
Reference ID: PHARMATUTOR-ART-1103
Introduction
Osteoporosis is a disease characterized by low bone mass and loss of bone tissue that may lead to weak and fragile bones. If you have osteoporosis, you have an increased risk for fractured bones (broken bones), particularly in the hip, spine, andwrist (1).
Osteoporosis is often considered to be a condition that frail elderly women develop. However, the damage from osteoporosis begins much earlier in life. Because peak bone density is reached at approximately 25 years of age, it is important to build strong bones by that age, so that the bones will remain strong later in life. Adequate calcium intake is an essential part of building strong bones.
In the United States, nearly 10 million people already have osteoporosis. Another 18 million people have low bone mass that places them at an increased risk for developing osteoporosis. As our population ages, these numbers will increase. About 80% of those with osteoporosis are women. Of people older than 50 years of age, one in two women and one in eight men are predicted to have an osteoporosis-related fracture in their lifetime (2).
According to the World Health Organization, the prevalence of osteoporosis among U.S. white women past menopause is estimated to be 14% in those 50-59 years of age, 22% in those 60-69 years of age, 39% in those 70-79 years of age, and 70% in those 80 years of age and older. Significant risk has been reported in people of all ethnic backgrounds. White and Asian racial groups, however, are at greatest risk (3).
Osteoporosis Symptoms
Early in the course of the disease, osteoporosis may cause no symptoms. Later, it may cause dull pain in the bones or muscles, particularly low back pain or neck pain.
Later in the course of the disease, sharp pains may come on suddenly. The pain may not radiate (spread to other areas); it may be made worse by activity that puts weight on the area, may be accompanied by tenderness, and generally begins to subside in one week. Pain may linger more than three months (4).
People with osteoporosis may not even recall a fall or other trauma that might cause a broken bone, such as in the spine or foot. Spinal compression fractures may result in loss of height with a stooped posture (called adowager's hump).
Fractures at other sites, commonly the hip or bones of the wrist, usually result from a fall.
Osteoporosis is characterized by low bone mass and deterioration of bone tissue. Osteoporosis is often known as the silent thief because bone loss occurs without symptoms. True osteoporosis causes bone pain and or spontaneous fractures of the spine, hip and wrist.
Osteoporosis is usually asymptomatic until fracture develops. Older persons with a fracture, especially spontaneous fracture of the spine, wrist or femur should be investigated for osteoporosis
[adsense:468x15:2204050025]
Prevalence
One in four women over the age of 50 has osteoporosis. One in eight men over 50 also has the disease. However, the disease can strike at any age. More women die each year as a result of osteoporotic fractures than from breast and ovarian cancer combined. A fifty-year-old woman has at least a 40 per cent risk of an osteoporotic fracture during the remainder of her life. Up to 20 per cent of individuals, who fracture a hip, die as a result of complications. Fifty per cent of those who survive remain permanently disabled (6).
Causes, incidence and risk factors
Osteoporosis is the most common type of bone disease.
Researchers estimate that about 1 out of 5 American women over the age of 50 have osteoporosis. About half of all women over the age of 50 will have a fracture of the hip, wrist, or vertebra (bones of the spine).
Osteoporosis occurs when the body fails to form enough new bone, when too much old bone is reabsorbed by the body, or both.
Calcium and phosphate are two minerals that are essential for normal bone formation. Throughout youth, your body uses these minerals to produce bones. If you do not get enough calcium, or if your body does not absorb enough calcium from the diet, bone production and bone tissues may suffer.
As you age, calcium and phosphate may be reabsorbed back into the body from the bones, which makes the bone tissue weaker. This can result in brittle, fragile bones that are more prone to fractures, even without injury (7).
Usually, the loss occurs gradually over years. Many times, a person will have a fracture before becoming aware that the disease is present. By the time a fracture occurs, the disease is in its advanced stages and damage is severe.
The leading causes of osteoporosis are a drop in estrogen in women at the time of menopause and a drop in testosterone in men. Women over age 50 and men over age 70 have a higher risk for osteoporosis (8).
Other causes include:
• Being confined to a bed
• Chronic rheumatoid arthritis, chronic kidney disease, eating disorders
• Taking corticosteroid medications (prednisone, methylprednisolone) every day for more than 3 months, or taking some antiseizure drugs
• Hyperparathyroidism
• Vitamin D deficiency
White women, especially those with a family history of osteoporosis, have a greater than average risk of developing osteoporosis. Other risk factors include:
• Absence of menstrual periods (amenorrhea) for long periods of time
• Drinking a large amount of alcohol
• Family history of osteoporosis
• History of hormone treatment for prostate cancer or breast cancer
• Low body weight
• Smoking
• Too little calcium in the diet
Symptoms
There are no symptoms in the early stages of the disease.
Symptoms occurring late in the disease include:
• Bone pain or tenderness
• Fractures with little or no trauma
• Loss of height (as much as 6 inches) over time
• Low back pain due to fractures of the spinal bones
• Neck pain due to fractures of the spinal bones
• Stooped posture or kyphosis, also called a "dowager's hump"
Signs and tests
Bone mineral density testing (specifically a densitometry or DEXA scan) measures how much bone you have. Your health care provider uses this test to predict your risk for bone fractures in the future. For information about when testing should be done, seebone density test.
A special type of spine CT that can show loss of bone mineral density, quantitative computed tomography (QCT), may be used in rare cases (9).
In severe cases, a spine or hip x-ray may show fracture or collapse of the spinal bones. However, simple x-rays of bones are not very accurate in predicting whether someone is likely to have osteoporosis.
You may need other blood and urine tests if your osteoporosis is thought to be due to a medical condition, rather than simply the usual bone loss seen with older age.
Treatment
The goals of osteoporosis treatment are to:
• Control pain from the disease
• Slow down or stop bone loss
• Prevent bone fractures with medicines that strengthen bone
• Minimize the risk of falls that might cause fractures
There are several different treatments for osteoporosis, including lifestyle changes and a variety of medications.
Medications are used to strengthen bones when:
• Osteoporosis has been diagnosed by a bone density study.
• Osteopenia (thin bones, but not osteoporosis) has been diagnosed by a bone density study, if a bone fracture has occurred.
BISPHOSPHONATES
Bisphosphonates are the primary drugs used to both prevent and treat osteoporosis in postmenopausal women.
• Bisphosphonates taken by mouth include alendronate (Fosamax), ibandronate (Boniva), and risedronate (Actonel). Most are taken by mouth, usually once a week or once a month.
• Bisphosphonates given through a vein (intravenously) are taken less often.
CALCITONIN
Calcitonin is a medicine that slows the rate of bone loss and relieves bone pain. It comes as a nasal spray or injection. The main side effects are nasal irritation from the spray form and nausea from the injectable form (10).
Calcitonin appears to be less effective than bisphosphonates.
HORMONE REPLACEMENT THERAPY
Estrogens or hormone replacement therapy (HRT) is rarely used anymore to prevent osteoporosis and are not approved to treat a woman who has already been diagnosed with the condition.
Sometimes, if estrogen has helped a woman, and she cannot take other options for preventing or treating osteoporosis, the doctor may recommend that she continue using hormone therapy. If you are considering taking hormone therapy to prevent osteoporosis, discuss the risks with your doctor (11).
PARATHYROID HORMONE
Teriparatide (Forteo) is approved for the treatment of postmenopausal women who have severe osteoporosis and are considered at high risk for fractures. The medicine is given through daily shots underneath the skin. You can give yourself the shots at home (12).
RALOXIFENE
Raloxifene (Evista) is used for the prevention and treatment of osteoporosis. Raloxifene is similar to the breast cancer drug tamoxifen. Raloxifene can reduce the risk of spinal fractures by almost 50%. However, it does not appear to prevent other fractures, including those in the hip. It may have protective effects against heart disease and breast cancer, though more studies are needed (13).
The most serious side effect of raloxifene is a very small risk of blood clots in the leg veins (deep venous thrombosis) or in the lungs (pulmonary embolus).
EXERCISE
Regular exercise can reduce the likelihood of bone fractures in people with osteoporosis. Some of the recommended exercises include:
• Weight-bearing exercises -- walking, jogging, playing tennis, dancing
• Resistance exercises -- free weights, weight machines, stretch bands
• Balance exercises -- tai chi, yoga
• Riding a stationary bicycle
• Using rowing machines
Avoid any exercise that presents a risk of falling, or high-impact exercises that may cause fractures.
DIET
Get at least 1,200 milligrams per day of calcium and 800 - 1,000 international units of vitamin D3. Vitamin D helps your body absorb calcium. Your doctor may recommend a supplement to give you the calcium and vitamin D you need (14).
Follow a diet that provides the proper amount of calcium, vitamin D, and protein. While this will not completely stop bone loss, it will guarantee that a supply of the materials the body uses to form and maintain bones is available.
High-calcium foods include:
• Cheese
• Ice cream
• Leafy green vegetables, such as spinach and collard greens
• Low-fat milk
• Salmon
• Sardines (with the bones)
• Tofu
• Yogurt
STOP UNHEALTHY HABITS
Quit smoking, if you smoke. Also limit alcohol intake. Too much alcohol can damage your bones, as well as put you at risk for falling and breaking a bone.
PREVENT FALLS
It is critical to prevent falls. Avoid sedating medications and remove household hazards to reduce the risk of fractures. Make sure your vision is good. Other ways to prevent falling include:
• Avoiding walking alone on icy days
• Using bars in the bathtub, when needed
• Wearing well-fitting shoes
MONITORING
Your response to treatment can be monitored with a series of bone mineral density measurements taken every 1 - 2 years (15).
Women taking estrogen should have routine mammograms, pelvic exams, and Pap smears.
RELATED SURGERIES
There are no surgeries for treating osteoporosis itself. However, a procedure called vertebroplasty can be used to treat any small fractures in your spinal column due to osteoporosis. It can also help prevent weak vertebrae from becoming fractured by strengthening the bones in your spinal column (16).
The procedure involves injecting a fast-hardening glue into the areas that are fractured or weak. A similar procedure, called kyphoplasty, uses balloons to widen the spaces that need the glue. (The balloons are removed during the procedure.)
Expectations (prognosis)
Medications to treat osteoporosis can help prevent fractures, but vertebrae that have already collapsed cannot be reversed (17).
Some persons with osteoporosis become severely disabled as a result of weakened bones. Hip fractures leave about half of patients unable to walk independently. This is one of the major reasons people are admitted to nursing homes (18).
Although osteoporosis is debilitating, it does not affect life expectancy.
Complications
• Compression fractures of the spine
• Disability caused by severely weakened bones
• Hip and wrist fractures
• Loss of ability to walk due to hip fractures
Calling your health care provider
Call your health care provider if you have symptoms of osteoporosis or if you wish to be screened for the condition.
Prevention
Calcium is essential for building and maintaining healthy bone. Vitamin D is also needed because it helps your body absorb calcium. Following a healthy, well-balanced diet can help you get these and other important nutrients throughout life. Building strong bones during childhood and adolescence can be the best defense against developing osteoporosis later. The average woman has acquired 98% of her skeletal mass by 30 years of age (19).
There are four steps to prevent osteoporosis. No one step alone is enough to prevent osteoporosis.
• Eat a balanced diet rich in calcium and vitamin D.
• Engage in weight-bearing exercise.
• Adopt a healthy lifestyle with no smoking or excessive alcohol intake.
• Take medication to improve bone density when appropriate.
WHO defines osteoporosis as a marked reduction in bone density, to differentiate it from osteopenia which refers to mild reduction in bone density. In osteoporosis, the bone density is more than 2.5 standard deviations below the young normal mean (20).
Exams and Tests
• The doctor will usually begin with a careful history to determine if you have osteoporosis or if you may be at risk for the disease. You will be asked a variety of questions regarding lifestyle and other conditions that you may have. The doctor will also ask if you have a family history of osteoporosis or a history of previous broken bones. Often blood tests are used to measure calcium, phosphorus, vitamin D, testosterone, and thyroid and kidney function.
• Based on a medical examination, the doctor may recommend a specialized test called a bone mineral density test that can measure bone density in various sites of the body. A bone mineral density test can detect osteoporosis before a fracture occurs and can predict future fractures. A bone mineral density test can also monitor the effects of treatment if the tests are performed a year or more apart and may help determine the rate of bone loss (21).
• Several different machines measure bone density. All are painless, noninvasive, and safe. They are becoming more readily available. In many testing centers, you don't even have to change into an examination robe. Central machines may measure density in the hip, spine, and total body. Peripheral machines may measure density in the finger, wrist, kneecap, shinbone, and heel.
• The DXA (dual-energy X-ray absorptiometry) measures the bone density of the spine, hip, or total body. With your clothes on, you simply lie on your back with your legs on a large block. The X-ray machine moves quickly over your lower spine and hip area.
• SXA (single-energy X-ray absorptiometry) is performed with a smaller X-ray machine that measure bone density at the heel, shin bone, and kneecap. Some machines use ultrasound waves pulsing through water to measure the bone density in your heel. You place your bare foot in a water bath, and your heel fits into a footrest as sound waves pass through your ankle. This is a simple way to screen large numbers of people quickly. You might find this type of screening device at a health fair. Bone loss at the heel may mean bone loss in the spine, hip, or elsewhere in the body. If bone loss is found in this test, you might be asked to have the DXA to confirm the results and get a better measurement of your bone density (22).
The result of the bone mineral density is compared to two standards, or norms, known as "age matched" and "young normal." The age-matched reading compares your bone mineral density to what is expected of someone of your age, sex, and size. The young normal reading compares your density to the optimal peak bone density of a healthy young adult of the same sex. The information from a bone mineral density test enables the doctor to identify where you stand in relation to others your age and to young adults (which is presumed to be your maximum bone density). Scores significantly lower than "young normal" indicate you have osteoporosis and are at risk for bone fractures. The results will also help the doctor to decide the best way to manage your bone health. For patients who have borderline results, an especially helpful new method of determining the 10-year probability of fracturing bone can be determined using a program called FRAX. This computation method is available online and takes into account all risk factors for a given individual to determine their personal risk for fracture and, therefore, need for treatment (23).
Osteoporosis Screening
Dual-energy x-ray absorptiometry (DEXA) scans the entire body and measures BMD. From the results, physicians assess the risk for fracture in the hip, spine, and wrist. DEXA produces a high resolution image, resulting in a precise BMD measurement. The level of radiation is low and the test takes less than 5 minutes. DEXA effectively monitors changes in bone density during treatment.
Quantitative computed tomography (QCT) measures bone density in the hip and spine and produces a three-dimensional image that shows true volume density. QCT has the capacity to isolate an area for testing. The radiation level in QCT is 10 times higher than in DEXA (24).
Peripheral bone density testing uses ultrasound to identify bone loss in a localized area such as the heel or hand.
BMD measurement is given as a T-score and a Z-score:
• T-score: the deviation from the mean bone density of healthy young adults of the same gender and ethnicity
• Z-score: the deviation from the mean bone density of adults of the same age and gender
The World Health Organization uses a T-score of 2.5 to define osteoporosis. A T-score between -1 and -2.5 indicates some bone loss (osteopenia) and a risk of osteoporosis. Every standard deviation below normal doubles the risk for fracture:
• -1 standard deviation equals 2 times the risk of fracture
• -2 standard deviation equals 4 times the risk of fracture
• -3 standard deviation equals 8 times the risk of fracture
The Z-score is used to classify the type of osteoporosis. A score below 1.5 indicatesprimary osteoporosis, which is age related. A score of 1.5 and higher indicatessecondary osteoporosis, which is associated with calcitonin imbalance, malabsorption conditions (e.g., celiac disease, cystic fibrosis, lactose intolerance),alcohol abuse, smoking, and the use of certain medications (25).
X-rays can only verify advanced bone loss, vertebral collapse, and bone fractures.
REVIEW OF LITERATURE
Osteoporosis is a disease of bones that leads to an increased risk of fracture. In osteoporosis the bone mineral density (BMD) is reduced, bone microarchitecture is deteriorating, and the amount and variety of proteins in bone is altered. Osteoporosis is defined by the World Health Organization (WHO) as a bone mineral density that is 2.5 standard deviations or more below the mean peak bone mass (average of young, healthy adults) as measured by DXA; the term "established osteoporosis" includes the presence of a fragility fracture. The disease may be classified as primary type 1, primary type 2, or secondary. The form of osteoporosis most common in women after menopause is referred to as primary type 1 or postmenopausal osteoporosis. Primary type 2 osteoporosis or senile osteoporosis occurs after age 75 and is seen in both females and males at a ratio of 2:1. Finally, secondary osteoporosis may arise at any age and affects men and women equally. This form of osteoporosis results from chronic predisposing medical problems or disease, or prolonged use of medications such as glucocorticoids, when the disease is called steroid- or glucocorticoid-induced osteoporosis (SIOP or GIOP) (26).
Osteoporosis risks can be reduced with lifestyle changes and sometimes medication; in people with osteoporosis, treatment may involve both. Lifestyle change includes diet and exercise, andpreventing falls. Medication includes calcium, vitamin D, bisphosphonates and several others. Fall-prevention advice includes exercise to tone deambulatory muscles, proprioception-improvement exercises; equilibrium therapies may be included. Exercise with its anabolic effect, may at the same time stop or reverse osteoporosis. Osteoporosis is a component of the frailty syndrome (27).
Signs and symptoms
Osteoporosis itself has no specific symptoms; its main consequence is the increased risk of bone fractures. Osteoporotic fractures are those that occur in situations where healthy people would not normally break a bone; they are therefore regarded as fragility fractures. Typical fragility fractures occur in the vertebral column, rib, hip and wrist (28).
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Fractures
The symptoms of a vertebral collapse ("compression fracture") are sudden back pain, often with radiculopathic pain (shooting pain due to nerve root compression) and rarely with spinal cord compression or cauda equina syndrome. Multiple vertebral fractures lead to a stooped posture, loss of height, and chronic pain with resultant reduction in mobility (29).
Fractures of the long bones acutely impair mobility and may require surgery. Hip fracture, in particular, usually requires prompt surgery, as there are serious risks associated with a hip fracture, such as deep vein thrombosis and a pulmonary embolism, and increased mortality (30). Fracture Risk Calculators assess the risk of fracture based upon several criteria, including BMD, age, smoking, alcohol usage, weight, and gender. Recognised calculators include FRAX and Dubbo.
Falls risk
The increased risk of falling associated with aging leads to fractures of the wrist, spine and hip. The risk of falling, in turn, is increased by impaired eyesight due to any cause (e.g. glaucoma, macular degeneration), balance disorder, movement disorders (e.g. Parkinson's disease), dementia, and sarcopenia (age-related loss of skeletal muscle). Collapse (transient loss of postural tone with or without loss of consciousness) leads to a significant risk of falls; causes of syncope are manifold but may include cardiac arrhythmias (irregular heart beat),vasovagal syncope, orthostatic hypotension (abnormal drop in blood pressure on standing up) and seizures. Removal of obstacles and loose carpets in the living environment may substantially reduce falls. Those with previous falls, as well as those with a gait or balance disorder, are most at risk (31).
Risk factors
Risk factors for osteoporotic fracture can be split between non-modifiable and (potentially) modifiable. In addition, there are specific diseases and disorders in which osteoporosis is a recognized complication. Medication use is theoretically modifiable, although in many cases the use of medication that increases osteoporosis risk is unavoidable. Caffeine is not a risk factor osteoporosis (32).
Nonmodifiable
The most important risk factors for osteoporosis are advanced age (in both men and women) and female gender; estrogen deficiency followingmenopause or oophorectomy is correlated with a rapid reduction in bone mineral density, while in men a decrease in testosterone levels has a comparable (but less pronounced) effect. While osteoporosis occurs in people from all ethnic groups, European or Asian ancestry predisposes for osteoporosis. Those with a family history of fracture or osteoporosis are at an increased risk; the heritability of the fracture as well as low bone mineral density are relatively high, ranging from 25 to 80 percent. There are at least 30 genes associated with the development of osteoporosis. Those who have already had a fracture are at least twice as likely to have another fracture compared to someone of the same age and sex. A small stature is also a non-modifiable risk factor associated with the development of osteoporosis (33).
Potentially modifiable
• Excess alcohol—small amounts of alcohol do not increase osteoporosis risk and may even be beneficial, but chronic heavy drinking (alcohol intake greater than 3 units/day), especially at a younger age, increases risk significantly.
• Vitamin D deficiency—low circulating Vitamin D is common among the elderly worldwide. Mild vitamin D insufficiency is associated with increased Parathyroid Hormone (PTH) production. PTH increases bone resorption, leading to bone loss. A positive association exists between serum 1,25-dihydroxycholecalciferol levels and bone mineral density, while PTH is negatively associated with bone mineral density (34). Tobacco smoking—tobacco smoking inhibits the activity of osteoblasts, and is an independent risk factor for osteoporosis. Smoking also results in increased breakdown of exogenous estrogen, lower body weight and earlier menopause, all of which contribute to lower bone mineral density.
• Malnutrition—nutrition has an important and complex role in maintenance of good bone. Identified risk factors include low dietary calciumand/or phosphorus, magnesium, zinc, boron, iron, fluoride, copper, vitamins A, K, E and C (and D where skin exposure to sunlight provides an inadequate supply). Excess sodium is a risk factor. High blood acidity may be diet-related, and is a known antagonist of bone. Some have identified low protein intake as associated with lower peak bone mass during adolescence and lower bone mineral density in elderly populations. Conversely, some have identified low protein intake as a positive factor, protein is among the causes of dietary acidity. Imbalance of omega 6 to omega 3 polyunsaturated fats is yet another identified risk factor.
• High protein diet—Research has found an association between diets high in animal protein and increased urinary calcium loss from the bones (35).
• Underweight/inactive—bone remodeling occurs in response to physical stress, and weight bearing exercise can increase peak bone mass achieved in adolescence. In adults, physical activity helps maintain bone mass, and can increase it by 1 or 2%. Conversely, physical inactivity can lead to significant bone loss. (Incidence of osteoporosis is lower in overweight people.)
• Excess physical activity—excessive exercise can lead to constant damage to the bones which can cause exhaustion of the structures as described above. There are numerous examples of marathon runners who developed severe osteoporosis later in life. In women, heavy exercise can lead to decreased estrogen levels, which predisposes to osteoporosis. In addition, intensive training without proper compensatory increased nutrition increases the risk (36).
• Heavy metals—a strong association between cadmium, lead and bone disease has been established. Low level exposure to cadmium is associated with an increased loss of bone mineral density readily in both genders, leading to pain and increased risk of fractures, especially in the elderly and in females. Higher cadmium exposure results in osteomalacia (softening of the bone).
• Soft drinks—some studies indicate that soft drinks (many of which contain phosphoric acid) may increase risk of osteoporosis; Others suggest soft drinks may displace calcium-containing drinks from the diet rather than directly causing osteoporosis.
Diseases and disorders
Many diseases and disorders have been associated with osteoporosis. For some, the underlying mechanism influencing the bone metabolism is straight-forward, whereas for others the causes are multiple or unknown (37).
• In general, immobilization causes bone loss (following the 'use it or lose it' rule). For example, localized osteoporosis can occur after prolonged immobilization of a fractured limb in a cast. This is also more common in active patients with a high bone turn-over (for example, athletes). Other examples include bone loss during space flight or in people who are bedridden or who use wheelchairs for various reasons (38).
• Hypogonadal states can cause secondary osteoporosis. These include Turner syndrome, Klinefelter syndrome, Kallmann syndrome,anorexia nervosa, andropause, hypothalamic amenorrhea or hyperprolactinemia. In females, the effect of hypogonadism is mediated by estrogen deficiency. It can appear as early menopause (<45 years) or from prolonged premenopausal amenorrhea (>1 year). A bilateraloophorectomy (surgical removal of the ovaries) or a premature ovarian failure cause deficient estrogen production. In males, testosterone deficiency is the cause (for example, andropause or after surgical removal of the testes) (39).
• Endocrine disorders that can induce bone loss include Cushing's syndrome, hyperparathyroidism, thyrotoxicosis, hypothyroidism, diabetes mellitus type 1 and 2, acromegaly and adrenal insufficiency. In pregnancy and lactation, there can be a reversible bone loss.
• Malnutrition, parenteral nutrition and malabsorption can lead to osteoporosis. Nutritional and gastrointestinal disorders that can predispose to osteoporosis include coeliac disease, Crohn's disease, lactose intolerance, surgery (after gastrectomy, intestinal bypass surgery or bowel resection) and severe liver disease (especially primary biliary cirrhosis). Patients with bulimia can also develop osteoporosis. Those with an otherwise adequate calcium intake can develop osteoporosis due to the inability to absorb calcium and/or vitamin D. Other micro-nutrients such as vitamin K or vitamin B12 deficiency may also contribute.
• Patients with rheumatologic disorders like rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus and polyarticular juvenile idiopathic arthritis are at increased risk of osteoporosis, either as part of their disease or because of other risk factors (notably corticosteroid therapy). Systemic diseases such as amyloidosis and sarcoidosis can also lead to osteoporosis.
• Renal insufficiency can lead to osteodystrophy (40). Hematologic disorders linked to osteoporosis are multiple myeloma and other monoclonal gammopathies, lymphoma and leukemia,mastocytosis, hemophilia, sickle-cell disease and thalassemia.
• Several inherited disorders have been linked to osteoporosis. These include osteogenesis imperfecta, Marfan syndrome, hemochromatosis, hypophosphatasia, glycogen storage diseases, homocystinuria, Ehlers-Danlos syndrome, porphyria, Menkes' syndrome, epidermolysis bullosa and Gaucher's disease.
• People with scoliosis of unknown cause also have a higher risk of osteoporosis. Bone loss can be a feature of complex regional pain syndrome. It is also more frequent in people with Parkinson's disease and chronic obstructive pulmonary disease (41).
Medication
• Certain medications have been associated with an increase in osteoporosis risk; only steroids and anticonvulsants are classically associated, but evidence is emerging with regard to other drugs.
• Steroid-induced osteoporosis (SIOP) arises due to use of glucocorticoids - analogous to Cushing's syndrome and involving mainly the axial skeleton. The synthetic glucocorticoid prescription drug prednisone is a main candidate after prolonged intake. Some professional guidelines recommend prophylaxis in patients who take the equivalent of more than 30 mg hydrocortisone (7.5 mg of prednisolone), especially when this is in excess of three months. Alternate day use may not prevent this complication.
• Barbiturates, phenytoin and some other enzyme-inducing antiepileptics - these probably accelerate the metabolism of vitamin D (42). L-Thyroxine over-replacement may contribute to osteoporosis, in a similar fashion as thyrotoxicosis does. This can be relevant in subclinical hypothyroidism.
• Several drugs induce hypogonadism, for example aromatase inhibitors used in breast cancer, methotrexate and other anti-metabolite drugs, depot progesterone and gonadotropin-releasing hormone agonists.
• Anticoagulants - long-term use of heparin is associated with a decrease in bone density, and warfarin (and related coumarins) have been linked with an increased risk in osteoporotic fracture in long-term use.
• Proton pump inhibitors - these drugs inhibit the production of stomach acid; it is thought that this interferes with calcium absorption. Chronic phosphate binding may also occur with aluminium-containing antacids.
• Thiazolidinediones (used for diabetes) - rosiglitazone and possibly pioglitazone, inhibitors of PPARγ, have been linked with an increased risk of osteoporosis and fracture.
• Chronic lithium therapy has been associated with osteoporosis (43).
Pathogenesis
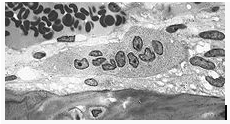
Osteoclast, with bone below it, showing typical distinguishing characteristics: a large cell with multiple nuclei and a "foamy" cytosol.
The underlying mechanism in all cases of osteoporosis is an imbalance between bone resorption and bone formation. In normal bone, there is constant matrix remodeling of bone; up to 10% of all bone mass may be undergoing remodeling at any point in time. The process takes place in bone multicellular units (BMUs) as first described by Frost in 1963. Bone is resorbed by osteoclastcells (which derive from the bone marrow), after which new bone is deposited by osteoblastcells (44).
The three main mechanisms by which osteoporosis develops are an inadequate peak bone mass(the skeleton develops insufficient mass and strength during growth), excessive bone resorption and inadequate formation of new bone during remodeling. An interplay of these three mechanisms underlies the development of fragile bone tissue. Hormonal factors strongly determine the rate of bone resorption; lack of estrogen (e.g. as a result of menopause) increases bone resorption as well as decreasing the deposition of new bone that normally takes place in weight-bearing bones. The amount of estrogen needed to suppress this process is lower than that normally needed to stimulate the uterus and breast gland. The α-form of the estrogen receptor appears to be the most important in regulating bone turnover.[8] In addition to estrogen, calcium metabolismplays a significant role in bone turnover, and deficiency of calcium and vitamin D leads to impaired bone deposition; in addition, theparathyroid glands react to low calcium levels by secreting parathyroid hormone (parathormone, PTH), which increases bone resorption to ensure sufficient calcium in the blood. The role of calcitonin, a hormone generated by the thyroid that increases bone deposition, is less clear and probably not as significant as that of PTH (45).
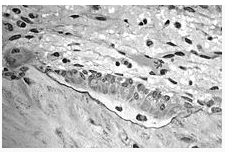
Osteoblasts, several displaying a prominent Golgi apparatus, actively synthesizing osteoid containing two osteocytes.
The activation of osteoclasts is regulated by various molecular signals, of which RANKL (receptor activator for nuclear factor κB ligand) is one of best studied. This molecule is produced by osteoblasts and other cells (e.g. lymphocytes), and stimulates RANK (receptor activator of nuclear factor κB). Osteoprotegerin (OPG) binds RANKL before it has an opportunity to bind to RANK, and hence suppresses its ability to increase bone resorption. RANKL, RANK and OPG are closely related to tumor necrosis factor and its receptors. The role of the wnt signalling pathway is recognized but less well understood. Local production of eicosanoids and interleukins is thought to participate in the regulation of bone turnover, and excess or reduced production of these mediators may underlie the development of osteoporosis (46).
Trabecular bone (or cancellous bone) is the sponge-like bone in the ends of long bones and vertebrae. Cortical bone is the hard outer shell of bones and the middle of long bones. Because osteoblasts and osteoclasts inhabit the surface of bones, trabecular bone is more active, more subject to bone turnover, to remodeling. Not only is bone density decreased, but the microarchitecture of bone is disrupted. The weaker spicules of trabecular bone break ("microcracks"), and are replaced by weaker bone. Common osteoporotic fracture sites, the wrist, the hip and the spine, have a relatively high trabecular bone to cortical bone ratio. These areas rely on trabecular bone for strength, and therefore the intense remodeling causes these areas to degenerate most when the remodeling is imbalanced. Around the ages of 30-35, cancellous or trabecular bone loss begins. Women may lose as much as 50%, while men lose about 30%.
Diagnosis
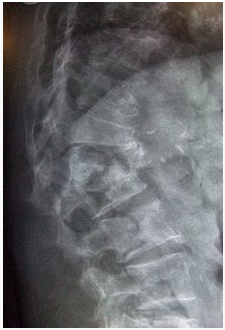
Multiple osteoporotic wedge fractures demonstrated on a lateral thoraco-lumbar spine X-ray
The diagnosis of osteoporosis can be made using conventional radiography and by measuring thebone mineral density (BMD). The most popular method of measuring BMD is dual energy x-ray absorptiometry (DXA or DEXA). In addition to the detection of abnormal BMD, the diagnosis of osteoporosis requires investigations into potentially modifiable underlying causes; this may be done with blood tests. Depending on the likelihood of an underlying problem, investigations forcancer with metastasis to the bone, multiple myeloma, Cushing's disease and other above-mentioned causes may be performed (47).
Conventional radiography
Conventional radiography is useful, both by itself and in conjunction with CT or MRI, for detecting complications of osteopenia (reduced bone mass; pre-osteoporosis), such as fractures; for differential diagnosis of osteopenia; or for follow-up examinations in specific clinical settings, such as soft tissue calcifications, secondary hyperparathyroidism, or osteomalacia in renal osteodystrophy. However, radiography is relatively insensitive to detection of early disease and requires a substantial amount of bone loss (about 30%) to be apparent on x-ray images (48).
The main radiographic features of generalized osteoporosis are cortical thinning and increased radiolucency. Frequent complications of osteoporosis are vertebral fractures for which spinal radiography can help considerably in diagnosis and follow-up. Vertebral height measurements can objectively be made using plain-film x-rays by using several methods such as height loss together with area reduction, particularly when looking at vertical deformity in T4-L4, or by determining a spinal fracture index that takes into account the number of vertebrae involved. Involvement of multiple vertebral bodies leads to kyphosis of the thoracic spine, obvious to the clinician as "dowager's hump."
Clinical decision rule
A number of clinical decision rules have been created to predict the risk of osteoporotic fractures. The QFracture score was developed in 2009 and is based on age, BMI, smoking status, alcohol use, rheumatoid arthritis, cardiovascular disease, type 2 diabetes, asthma, use of tricyclic antidepressants or corticosteroids, liver disease, and a history of falls in men. In women hormone replacement therapy, parental history of osteoporosis, gastrointestinal malabsorption, and menopausal symptoms are also taken into account. A website is available to help apply this score (49).
Dual energy X-ray absorptiometry
Dual energy X-ray absorptiometry (DXA, formerly DEXA) is considered the gold standard for the diagnosis of osteoporosis. Osteoporosis is diagnosed when the bone mineral density is less than or equal to 2.5 standard deviations below that of a young adult reference population. This is translated as a T-score. The World Health Organization has established the following diagnostic guidelines:
• T-score -1.0 or greater is "normal"
• T-score between -1.0 and -2.5 is "low bone mass" (or "osteopenia")
• T-score -2.5 or below is osteoporosis
When there has also been an osteoporotic fracture (also termed "low trauma-fracture" or "fragility fracture"), defined as one that occurs as a result of a fall from a standing height, the term "severe or established" osteoporosis is used (50).
The International Society for Clinical Densitometry takes the position that a diagnosis of osteoporosis in men under 50 years of age should not be made on the basis of densitometric criteria alone. It also states that for pre-menopausal women, Z-scores (comparison with age group rather than peak bone mass) rather than T-scores should be used, and that the diagnosis of osteoporosis in such women also should not be made on the basis of densitometric criteria alone.
Other measuring tools
Quantitative CT differs from DXA in that it gives separate estimates of BMD for trabecular and cortical bone and reports precise volumetric mineral density in mg/cm3 rather than BMD's relative Z score. Among QCT's advantages: it can be performed at axial and peripheral sites, is sensitive to change over time, can analyze a region of any size or shape, excludes irrelevant tissue such as fat, muscle, and air, and does not require knowledge of the patient's subpopulation in order to create a clinical score (e.g. the Z-score of all females of a certain age). Among QCT's disadvantages: it requires a high radiation dose, CT scanners are large and expensive, and because its practice has been less standardized than BMD, its results are more operator-dependent. Peripheral QCT has been introduced to improve upon the limitations of DXA and QCT (51).
Quantitative ultrasound has many advantages in assessing osteoporosis. The modality is small, no ionizing radiation is involved, measurements can be made quickly and easily, and the cost of the device is low compared with DXA and QCT devices. The calcaneus is the most common skeletal site for quantitative ultrasound assessment because it has a high percentage of trabecular bone that is replaced more often than cortical bone, providing early evidence of metabolic change. Also, the calcaneus is fairly flat and parallel, reducing repositioning errors. The method can be applied to children, neonates, and preterm infants, just as well as to adults. Once microimaging tools to examine specific aspects of bone quality are developed, it is expected that quantitative ultrasound will be increasingly used in clinical practice (52).
Screening
The U.S. Preventive Services Task Force (USPSTF) recommended in 2002 that all women 65 years of age or older should be screened with bone densitometry. The Task Force recommends screening women of any age with increased risk factors that puts them at risk equivalent to a 65 year old without additional risk factors. The best risk factor for indicating increased risk is lower body weight (weight < 70 kg), with less evidence for smoking or family history. There was insufficient evidence to make recommendations about the optimal intervals for repeated screening and the appropriate age to stop screening. Clinical prediction rules are available to guide selection of women ages 60–64 for screening. The Osteoporosis Risk Assessment Instrument (ORAI) may be the most sensitive strategy (53).
Regarding the screening of men, a cost-analysis study suggests that screening may be "cost-effective for men with a self-reported prior fracture beginning at age 65 years and for men 80 years and older with no prior fracture". Also cost-effective is the screening of adult men from middle age on to detect any significant decrease in testosterone levels, say, below 300 (54).
Prevention
Methods to prevent osteoporosis include changes of lifestyle. However, there are medications that can be used for prevention as well. As a different concept there are osteoporosis ortheses which help to prevent spine fractures and support the building up of muscles. Fall preventioncan help prevent osteoporosis complications (55).
Lifestyle
Lifestyle prevention of osteoporosis is in many aspects inversions from potentially modifiable risk factors. As tobacco smoking and unsafealcohol intake have been linked with osteoporosis, smoking cessation and moderation of alcohol intake are commonly recommended in the prevention of osteoporosis. Many other risk factors, some modifiable and others non modifiable such as genetic may be involved in osteoporosis.
Exercise
Achieving a higher peak bone mass through exercise and proper nutrition during adolescence is important for the prevention of osteoporosis. Exercise and nutrition throughout the rest of the life delays bone degeneration. Jogging, walking, or stair climbing at 70-90% of maximum effort three times per week, along with 1,500 mg of calcium per day, increased bone density of the lumbar (lower) spine by 5% over nine months. Individuals already diagnosed with osteopenia or osteoporosis should discuss their exercise program with their physician to avoid fractures (56).
Nutrition
Proper nutrition includes a diet sufficient in calcium and vitamin D. Patients at risk for osteoporosis (e.g. steroid use) are generally treated with vitamin D and calcium supplements and often with bisphosphonates. Vitamin D supplementation alone does not prevent fractures, and always needs to be combined with calcium. Calcium supplements come in two forms: calcium carbonate and calcium citrate. Due to its lower cost, calcium carbonate is often the first choice, however it needs to be taken with food to maximize absorption. Calcium citrate is more expensive, but it is better absorbed than calcium carbonate and can be taken without food. In addition, patients who are taking proton pump inhibitors or H2 blockers do not absorb calcium carbonate well; calcium citrate is the supplement of choice in this population. Inrenal disease, more active forms of Vitamin D such as cholecalciferol or (1,25-dihydroxycholecalciferol or calcitriol which is the main biologically active form of vitamin D) is used, as the kidney cannot adequately generate calcitriol from calcidiol (25-hydroxycholecalciferol) which is the storage form of vitamin D.In vitamin D assays, vitamin D2 (ergocalitrol) is not accurately measured, therefore vitamin D3(cholecalciferol) is recommended for supplementation (57).
High dietary protein intake increases calcium excretion in urine and has been linked to increased risk of fractures in research studies. Other investigations have shown that protein is required for calcium absorption, but that excessive protein consumption inhibits this process. No interventional trials have been performed on dietary protein in the prevention and treatment of osteoporosis.
Medication
Just as for treatment, bisphosphonate can be used in cases of very high risk. Other medicines prescribed for prevention of osteoporosis include raloxifene, a selective estrogen receptor modulator (SERM).
Estrogen replacement therapy remains a good treatment for prevention of osteoporosis but, at this time, is not recommended unless there are other indications for its use as well. There is uncertainty and controversy about whether estrogen should be recommended in women in the first decade after the menopause.
In hypogonadal men testosterone has been shown to give improvement in bone quantity and quality, but, as of 2008, there are no studies of the effects on fractures or in men with a normal testosterone level (58).
Treatment
There are several medications used to treat osteoporosis, depending on gender. Medications themselves can be classified as antiresorptive or bone anabolic agents. Antiresorptive agents work primarily by reducing bone resorption, while bone anabolic agents build bone rather than inhibit resorption. Lifestyle changes are an important aspect of treatment. A major problem is gaining long-term adherence to therapy from patients with osteoporosis. Fifty percent of patients do not take their medications and most discontinue within 1 year.
Antiresorptive agents
• Bisphosphonates
Bisphosphonates are the main pharmacological measures for treatment. However, newer drugs have appeared in the 1990s, such as teriparatide and strontium ranelate.
In confirmed osteoporosis, bisphosphonate drugs are the first-line treatment in women. The most often prescribed bisphosphonates are presently sodium alendronate (Fosamax) 10 mg a day or 70 mg once a week, risedronate (Actonel) 5 mg a day or 35 mg once a week and/or ibandronate (Boniva) once a month (59).
A 2007 manufacturer-supported study suggested that in patients who had suffered a low-impact hip fracture, annual infusion of 5 mgzoledronic acid reduced risk of any fracture by 35% (from 13.9 to 8.6%), vertebral fracture risk from 3.8% to 1.7% and non-vertebral fracture risk from 10.7% to 7.6%. This study also found a mortality benefit: after 1.9 years, 9.6% of the study group (as opposed to 13.3% of the control group) had died of any cause, indicating a mortality benefit of 28%. There are currently no studies which examine the efficacy or side-effects of zoledronic acid past the three-year period.
Oral bisphosphonates are relatively poorly absorbed, and must therefore be taken on an empty stomach, with no food or drink to follow for the next 30 minutes. They are associated with inflammation of the esophagus (esophagitis) and are therefore sometimes poorly tolerated; weekly or monthly administration (depending on the preparation) decreases likelihood of esophagitis, and is now standard. Although intermittent dosing with the intravenous formulations such as zolendronate (zoledronic acid) avoids oral tolerance problems, these agents are implicated at higher rates in a rare but severe bone disease called osteonecrosis of the jaw. For this reason, oral bisphosphonate therapy is probably to be preferred, and doctors now recommend that any needed remedial dental work be done before treatment begins (60).
• Estrogen analogs
Estrogen replacement therapy remains a good treatment for prevention of osteoporosis but, at this time, is not recommended unless there are other indications for its use as well. There is uncertainty and controversy about whether estrogen should be recommended in women in the first decade after the menopause.
In hypogonadal men testosterone has been shown to give improvement in bone quantity and quality, but, as of 2008, there are no studies of the effects on fractures or in men with a normal testosterone level.
• Raloxifene
Selective Estrogen Receptor Modulators (SERMs) are a class of medications that act on the estrogen receptors throughout the body in a selective manner. Normally, bone mineral density (BMD) is tightly regulated by a balance between osteoblast and osteoclast activity in the trabecular bone. Estrogen has a major role in regulation of the bone formation-resorption equilibrium, as it stimulates osteoblast activity. Some SERMs such as raloxifene, act on the bone by slowing bone resorption by the osteoclasts. Raloxifene has the added advantage of reducing the risk of invasive breast cancer. SERMs have been proven effective in clinical trials.
• Calcitonin
Calcitonin works by directly inhibiting osteoclast activity via the calcitonin receptor. Calcitonin receptors have been identified on the surface of osteoclasts. Calcitonin directly induces inhibition of osteoclastic bone resorption by affecting actin cytoskeleton which is needed for the osteoclastic activity.
Bone anabolic agents
• Teriparatide
Recently, teriparatide (Forteo, recombinant parathyroid hormone residues 1–34) has been shown to be effective in osteoporosis. It acts like parathyroid hormone and stimulates osteoblasts, thus increasing their activity. It is used mostly for patients with established osteoporosis (who have already fractured), have particularly low BMD or several risk factors for fracture or cannot tolerate the oral bisphosphonates. It is given as a daily injection with the use of a pen-type injection device. In some countries, Teriparatide is licensed to be used for treatment only if bisphosphonates have failed or are contraindicated. (In the US, this restriction has not been imposed by the FDA.) Patients with previous radiation therapy, or Paget's disease, or young patients, should avoid this medication.
• Calcium salts
Calcium salts come as water insoluble and soluble formulations. Calcium carbonate is the primary water insoluble drug, while calcium citrate, lactate, and gluconate are water soluble. Calcium carbonate's absorption is improved in acidic conditions, while the water soluble salts are relatively unaffected by acidic conditions.
• Sodium fluoride
Sodium fluoride treatment in patients with osteoporosis has been shown to cause skeletal changes such as pronounced bone density with increased number and thickness of trabeculae, cortical thickening, and partial obliteration of the medullary space.
Other agents
• RANKL inhibitors
Denosumab is a fully human monoclonal antibody that mimics the activity of osteoprotegerin. It binds to RANKL, thereby preventing RANKL from interacting with RANK and reducing its bone resorption. It was approved for use in the treatment of osteoporosis by the European Commission on May 28, 2010 and by the United States Food and Drug Administration on June 2, 2010.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
• Strontium ranelate
Oral strontium ranelate is an alternative oral treatment, belonging to a class of drugs called "dual action bone agents" (DABAs) by its manufacturer. It has proven efficacy, especially in the prevention of vertebral fracture. In laboratory experiments, strontium ranelate was noted to stimulate the proliferation of osteoblasts, as well as inhibiting the proliferation of osteoclasts.
Strontium ranelate is taken as a 2 g oral suspension daily, and is licenced for the treatment of osteoporosis to prevent vertebral and hip fracture. Strontium ranelate has side effect benefits over the bisphosphonates, as it does not cause any form of upper GI side effect, which is the most common cause for medication withdrawal in osteoporosis. In studies a small increase in the risk ofvenous thromboembolism was noted, the cause for which has not been determined. This suggests it may be less suitable in patients at risk for thrombosis for different reasons. The uptake of (heavier) strontium in place of calcium into bone matrix results in a substantial and disproportionate increase in bone mineral density as measured on DXA scanning, making further followup of bone density by this method harder to interpret for strontium treated patients. A correction algorithm has been devised.
Although strontium ranelate is effective, it is not approved for use in the United States yet. However, strontium citrate is available in the US from several well-known vitamin manufacturers. Most researchers believe that strontium is safe and effective no matter what form it is used. The ranelate form is simply a device invented by the Servier company of France so that they could patent their version of strontium.
Strontium, no matter what the form, must be water-soluble and ionized in the stomach acid. Strontium is then protein-bound for transport from the intestinal tract into the blood stream. Unlike drugs like sodium alendronate (Fosamax), strontium doesn't inhibit bone recycling and, in fact, may produce stronger bones. Studies have shown that after five years alendronate may even cause bone loss, while strontium continues to build bone during lifetime use.
Strontium must not be taken with food or calcium-containing preparations as calcium competes with strontium during uptake. However, it is essential that calcium, magnesium, and vitamin D in therapeutic amounts must be taken daily, but not at the same time as strontium. Strontium should be taken on an empty stomach at night.
Nutrition
• Calcium
Calcium is required to support bone growth, bone healing and maintain bone strength and is one aspect of treatment for osteoporosis. Recommendations for calcium intake vary depending country and age; for individuals at higher risk of osteoporosis (after fifty years of age) the amount recommended by US health agencies is 1,200 mg per day. Calcium supplements can be used to increase dietary intake, and absorption is optimized through taking in several small (500 mg or less) doses throughout the day. The role of calcium in preventing and treating osteoporosis is unclear — some populations with extremely low calcium intake also have extremely low rates of bone fracture, and others with high rates of calcium intake through milk and milk products have higher rates of bone fracture. Other factors, such as protein, salt and vitamin D intake, exercise and exposure to sunlight, can all influence bone mineralization, making calcium intake one factor among many in the development of osteoporosis. In the report of WHO (World Health Organization) in 2007, because calcium is consumed by an acid load with food, it influences osteoporosis.
A meta-analysis of randomized controlled trials involving calcium and calcium plus vitamin D supported the use of high levels of calcium (1,200 mg or more) and vitamin D (800 IU or more), though outcomes varied depending on which measure was used to assess bone health (rates of fracture versus rates of bone loss). The meta-analysis, along with another study, also supported much better outcomes for patients with high compliance to the treatment protocol. In contrast, despite earlier reports in improved high density lipoprotein (HDL, "good cholesterol") in calcium supplementation, a possible increase in the rate of myocardial infarction (heart attack) was found in a study in New Zealand in which 1471 women participated. If confirmed, this would indicate that calcium supplementation in women otherwise at low risk of fracture may cause more harm than good.
Vitamin D
Several studies have shown that a high intake of vitamin D reduces fractures in the elderly, The Women's Health Initiative found that though calcium plus vitamin D did increase bone density by 1% but it did not affect hip fracture. It did increase formation of kidney stonesby 17%.This study has been criticised for using an inadequate dose of vitamin D (400 U) and for allowing the control arm to take supplemental vitamin D.
Exercise
Multiple studies have shown that aerobics, weight bearing, and resistance exercises can all maintain or increase BMD in postmenopausal women. Many researchers have attempted to pinpoint which types of exercise are most effective at improving BMD and other metrics of bone quality, however results have varied. The BEST (Bone-Estrogen Strength Training) Project at the University of Arizona identified six specific weight training exercises that yielded the largest improvements in BMD; this project suggests squat, military press, lat pulldown, leg press, back extension, and seated row, with three weight training sessions a week of two sets of each exercise, alternating between moderate (6-8 reps at 70% of 1-rep max) and heavy (4-6 reps at 80% of 1-rep max). One year of regular jumping exercises appears to increase the BMD and moment of inertia of the proximal tibia in normal postmenopausal women. Treadmill walking, gymnastic training, stepping, jumping, endurance, and strength exercises all resulted in significant increases of L2-L4 BMD in osteopenic postmenopausal women. Strength training elicited improvements specifically in distal radius and hip BMD. Exercise combined with other pharmacological treatments such as hormone replacement therapy (HRT) has been shown to increases BMD more than HRT alone.
Additional benefits for osteoporotic patients other than BMD increase include improvements in balance, gait, and a reduction in risk of falls (61).
Prognosis
Although osteoporosis patients have an increased mortality rate due to the complications of fracture, it is rarely lethal (62).
Hip fractures can lead to decreased mobility and an additional risk of numerous complications (such as deep venous thrombosis and/or pulmonary embolism, pneumonia). The 6-month mortality rate following hip fracture is approximately 13.5%, and a substantial proportion (almost 13%) of people who have suffered a hip fracture need total assistance to mobilize after a hip fracture (63).
Vertebral fractures, while having a smaller impact on mortality, can lead to severe chronic pain of neurogenic origin, which can be hard to control, as well as deformity. Though rare, multiple vertebral fractures can lead such severe hunch back (kyphosis) that the resulting pressure on internal organs can impair one's ability to breathe (64).
Apart from risk of death and other complications, osteoporotic fractures are associated with a reduced health-related quality of life (65).
Epidemiology
Osteoporosis is a major public health threat which afflicts 55% of Americans aged 50 and above. Of these, approximately 80% are women. It is estimated that 1 in 3 women and 1 in 12 men over the age of 50 worldwide have osteoporosis. It is responsible for millions of fractures annually, mostly involving the lumbar vertebrae, hip, and wrist. Fragility fractures of ribs are also common in men (66).
Hip fractures
Main article: hip fractures
Hip fractures are responsible for the most serious consequences of osteoporosis. In the United States, more than 250,000 hip fractures annually are attributable to osteoporosis. It is estimated that a 50-year-old white woman has a 17.5% lifetime risk of fracture of the proximal femur. The incidence of hip fractures increases each decade from the sixth through the ninth for both women and men for all populations. The highest incidence is found among men and women ages 80 or older (67).
Vertebral fractures
Between 35-50% of all women over 50 had at least one vertebral fracture. In the United States, 700,000 vertebral fractures occur annually, but only about a third are recognized. In a series of 9704 of women aged 68.8 on average studied for 15 years, 324 had already suffered a vertebral fracture at entry into the study; 18.2% developed a vertebral fracture, but that risk rose to 41.4% in women who had a previous vertebral fracture (68).
Wrist
In the United States, 250,000 wrist fractures annually are attributable to osteoporosis. Wrist fractures are the third most common type of osteoporotic fractures. The lifetime risk of sustaining a Colles' fracture is about 16% for white women. By the time women reach age 70, about 20% have had at least one wrist fracture (69).
Rib Fractures
Fragility fractures of the ribs are common in men as young as age thirty-five on. These are often overlooked as signs of osteoporosis as these men are often physically active and suffer the fracture in the course of physical activity. An example would be as a result of falling while water skiing or jet skiing. However, a quick test of the individual's testosterone level following the diagnosis of the fracture will readily reveal whether that individual might be at risk (70).
History
The link between age-related reductions in bone density and fracture risk goes back at least to Astley Cooper, and the term "osteoporosis" and recognition of its pathological appearance is generally attributed to the French pathologist Jean Lobstein. The American endocrinologist Fuller Albright linked osteoporosis with the postmenopausal state. Bisphosponates, which revolutionized the treatment of osteoporosis, were discovered in the 1960s (71).
Awareness
Various organizations have been established to raise awareness on osteoporosis.
The National Osteoporosis Society, established in 1986, is a United Kingdom charity dedicated to improving the diagnosis, prevention and treatment of osteoporosis.
The National Osteoporosis Foundation (headquartered in Washington, D.C., US) seeks to prevent osteoporosis and related fractures, to promote lifelong bone health, to help improve the lives of those affected by osteoporosis and to find a cure through programs of awareness, advocacy, public and health professional education and research (72).
The International Osteoporosis Foundation (IOF) (headquartered in Nyon, Switzerland) functions as a global alliance of patient, medical and research societies, scientists, health care professionals, and international companies concerned about bone health (73).
The Orthopaedic Research Society (headquartered in Rosemont, IL, US) is a research and professional development society that has emphasized osteoporosis research, treatment and prevention for many years.
Aim and Scope
Aim is to understand the interaction between the Calcium Sensing Receptor and the Ranalic acids and to generate a three dimensional 3D) model of the Calcium Sensing Receptor based on the crystal structure of 2E4U chain A, With the aid of the molecular mechanics and molecular dynamics methods, scope is to show interaction of protein, ligand i.e.Calcium Sensing Receptor and the Ranalic acids.
Hence Ranalic acids can make drug more efficient than pervious.
Materials & Methods
Materials
Software’s and servers
Blast
BLAST searches for high scoring sequence alignments between the query sequence and sequences in the database using a heuristic approach that approximates the Smith-Waterman algorithm. The exhaustive Smith-Waterman approach is too slow for searching large genomic databases such as GenBank. Therefore, the BLAST algorithm uses a heuristic approach that is slightly less accurate than Smith-Waterman but over 50 times faster. The speed and relatively good accuracy of BLAST are the key technical innovation of the BLAST programs and arguably why the tool is the most popular bioinformatics search tool
BLAST is actually a family of programs (all included in the blastall executable). The following are some of the programs, ranked mostly in order of importance:
a) Nucleotide-nucleotide BLAST (blastn): This program, given a DNA query, returns the most similar DNA sequences from the DNA database that the user specifies.
b) Protein-protein BLAST (blastp): This program, given a protein query, returns the most similar protein sequences from the protein database that the user specifies.
c) Position-Specific Iterative BLAST (PSI-BLAST): One of the more recent BLAST programs, this program is used for finding distant relatives of a protein. First, a list of all closely related proteins is created. Then these proteins are combined into a "profile" that is a sort of average sequence. A query against the protein database is then run using this profile, and a larger group of proteins found. This larger group is used to construct another profile, and the process is repeated. By including related proteins in the search PSI-BLAST is much more sensitive in picking up distant evolutionary relationships than the standard protein-protein BLAST.
d) Nucleotide 6-frame translation-protein (blastx): This program compares the six-frame conceptual translation products of a nucleotide query sequence (both strands) against a protein sequence database.
e) Nucleotide 6-frame translation-nucleotide 6-frame translation (tblastx): This program is the slowest of the BLAST family. It translates the query nucleotide sequence in all six possible frames and compares it against the six-frame translations of a nucleotide sequence database. The purpose of tblastx is to find very distant relationships between nucleotide sequences.
f) Protein-nucleotide 6-frame translation (tblastn): This program compares a protein query against the six-frame translations of a nucleotide sequence database.
g) Large numbers of query sequences (megablast): When comparing large numbers of input sequences via the command-line BLAST, "megablast" is much faster than running BLAST multiple times. It basically concatenates many input sequences together to form a large sequence before searching the BLAST database, then post-analyze the search results to glean individual alignments and statistical values".
Homology modelling
All homology-modeling methods consist of the following four steps:
(i) Template selection;
(ii) Target template alignment;
(iii) Model building; and
(iv) Evaluation.
These steps can be iteratively repeated, until a satisfying model structure is achieved. Several different techniques for model building have been developed. The SWISS-MODEL server approach can be described as rigid fragment assembly is outlined briefly.
Template selection
The SWISS-MODEL server template library Ex: PDB is extracted from the PDB. In order to allow a stable and automated workflow of the server, the PDB coordinate files are split into individual protein chains and unreliable entries, e.g. theoretical models and low quality structures providing only C coordinates, are removed. Additional information useful for template selection is gathered and added to the file header, e.g. probable quaternary structure, quality indicators like empirical force field energy or ANOLEA mean force potential scores. To select templates for a given protein, the sequences of the template structure library are searched. If these templates cover distinct regions of the target sequence, the modeling process will be split into separate independent batches.
Alignment
Up to five template structures per batch are superposed using an iterative least squares algorithm. A structural alignment is generated after removing incompatible templates, i.e. omitting structures with high C root mean square deviations to the first template. A local pair-wise alignment of the target sequence to the main template structures is calculated, followed by a heuristic step to improve the alignment for modeling purposes. The placement of insertions and deletions is optimized considering the template structure context. In particular, isolated residues in the alignment (‘islands’) are moved to the flanks to facilitate the loop building process.
Model building
To generate the core of the model, the backbone atom positions of the template structure are averaged. The templates are thereby weighted by their sequence similarity to the target sequence, while significantly deviating atom positions are excluded. The template coordinates cannot be used to model regions of insertions or deletions in the target-template alignment. To generate those parts, an ensemble of fragments compatible with the neighboring stems is constructed using constraint space programming (CSP). The best loop is selected using a scoring scheme, which accounts for force field energy, steric hindrance and favorable interactions like hydrogen bond formation. If no suitable loop can be identified, the flanking residues are included to the rebuilt fragment to allow for more flexibility. In cases where CSP does not give a satisfying solution and for loops above 10 residues, a loop library derived from experimental structures is searched to find compatible loop fragments.
Side chain modeling
The reconstruction of the model side chains is based on the weighted positions of corresponding residues in the template structures. Starting with conserved residues, the model side chains are built by iso-sterically replacing template structure side chains. Possible side chain conformations are selected from a backbone dependent rotamer library, which has been constructed carefully taking into account the quality of the source structures. A scoring function assessing favorable interactions (hydrogen bonds, disulfide bridges) and unfavorably close contacts is applied to select the most likely conformation.
Verify 3D
Verify3D Structure Evaluation Server is a tool designed to help in the refinement of crystallographic structures. It will provide a visual analysis of the quality of a putative crystal structure for a protein. Verify3D expects this crystal structure to be submitted in PDB format. Verify3D works best on proteins with at least 100 residues.
Ramachandran plot
A Ramachandran plot developed by G.N.Ramachandran is a way to visualize dihedral angles φ against ψ of amino acid residues in protein structure. It shows the possible conformations of φ and ψ angles for a polypeptide A scatter plot showing the disposition of back bone φ and ψ torsion angles for each residue in a protein or set of proteins. Certain combinations of φ and ψ angles are preferred strongly are reported in a series of residues and these patterns are easily detected.
Glycine has a hydrogen atom, instead of a methyl group at the β position. Hence it is least restricted and this is apparent in the Ramachandran plot for Glycine for which the allowable area is considerably larger. In contrast, the Ramachandran plot for Proline shows only a very limited number of possible combinations of ψ and φ. Since bond length and angles are fairly invariant in the known protein structures, the key to protein folding lies in the torsion angles of the backbone. The quality of the structures, and of Ramachandran plot statistics in particular, was notably improved while preserving the agreement with the experimental constraints the compatibility scores above zero.
Energy minimization
Deviations in the protein structure geometry, which have been introduced by the modeling algorithm when joining rigid fragments are regularized in the last modeling step by steepest descent energy minimization using the NAMD/VMD molecular dynamics simulation package. Empirical force fields are useful to detect parts of the model with conformational errors. Energy minimization or molecular dynamics methods are in general not able to improve the accuracy of the models, and are used in SWISS-MODEL only to regularize the structure. However, the successful application of restricted molecular dynamics for improving homology models has recently been reported for a few test cases. To derive more general rules of engagement of molecular dynamics, further systematic experiments have to be conducted.
The four modeling steps — template superposition, target-template alignment, model building and energy minimization — have to be implemented in the program.
CASTp
Computed Atlas of Surface Topography of proteins (CASTp) provides an online resource for locating, delineating and measuring concave surface regions on three-dimensional structures of proteins. These include pockets located on protein surfaces and voids buried in the interior of proteins. The measurement includes the area and volume of pocket or void by solvent accessible surface model (Richards' surface) and by molecular surface model (Connolly's surface), all calculated analytically. CASTp can be used to study surface features and functional regions of proteins. CASTp includes a graphical user interface, flexible interactive visualization, as well as on-the-fly calculation for user uploaded structures.
ChemSketch
ACD/ChemSketch is an integrated software package from Advanced Chemistry Development Inc. for drawing chemical structures, reactions, schematic diagrams and designing other chemistry-related reports and presentations. Structure mode for drawing chemical structures and calculating their properties.
Docking
Docking studies are computational techniques for the exploration of the possible binding modes of a substrate to a given receptor, enzyme or other binding site. Docking studies have become nearly indispensable for study of macromolecular structures and interactions. Mechanical model construction requires heroic patience and endurance to complete a structure which may contain several thousand atoms while computer graphics can build and display in seconds. Macromolecular modeling by Docking studies provides most detailed possible view of drug-receptor interaction and has created a new rational approach to drug design where the structure of drug is designed based on its fit to three dimensional structures of receptor site, rather than by analogy to other active structures of random leads.
Methodology
3D model building
The initial model of Calsium Sensing Receptor (CASR) was built by using homology-modeling methods and the MODELLER software; a program for comparative protein structure modeling optimally satisfying spatial restraints derived from the alignment and expressed as probability density functions (pdfs) for the features restrained. The pdfs restrain Cα-Cα distances, main-chain N-O distances, main-chain and side-chain dihedral angles. The 3D model of a protein is obtained by optimization of the molecular pdf such that the model violates the input restraints as little as possible. The molecular pdf is derived as a combination of pdfs restraining individual spatial features of the whole molecule.
The optimization procedure is a variable target function method that applies the conjugate gradients algorithm to positions of all non-hydrogen atoms. The query sequence from Homo sapiens was submitted to domain fishing server casr. The predicted domain was searched to find out the related protein structure to be used as a template by the BLAST (Basic Local Alignment Search Tool) program against PDB (Protein Databank). Sequence that showed maximum identity with high score and less e-value were aligned and was used as a reference structure to build a 3D model for Calsium Sensing Receptor. The sequence of Calsium Sensing Receptor (Q2F3K4) was obtained from NCBI.
The co-ordinates for the structurally conserved regions (SCRs) for Calsium Sensing Receptor were assigned from the template using multiple sequence alignment, based on the Needleman-Wunsch algorithm. The structure having the least modeller objective function, obtained from the modeller was improved by molecular dynamics and equilibration methods using NAMD 2.5 software using CHARMM27 force field for lipids and proteins along with the TIP3P model for water. The energy of the structure was minimized with 1, 00, 00 steps. A cutoff of 12 Å (switching function starting at 10 Å) for van der Waals interactions was assumed. No periodic boundary conditions were included in this study. An integration time step of 2 fs was used, permitting a multiple time-stepping algorithm to be employed in which interactions involving covalent bonds were computed every time step, short-range nonbonded interactions were computed every two time steps, and long-range electrostatic forces were computed every four time steps.
The pair list of the nonbonded interaction was recalculated every ten time steps with a pair list distance of 13.5 Å. The short-range nonbonded interactions were defined as van der Waals and electrostatics interactions between particles within 12 Å. A smoothing function was employed for the van der Waals interactions at a distance of 10 Å. CHARMM27 [force-field parameters were used in all simulations in this study. The equilibrated system was simulated for 1 ps with a 500 kcal/mol/Å2 restraint on the protein backbone under 1 atm constant pressure and 310 K constant temperature (NPT) and the Langevin damping coefficient was set to 5 ps unless otherwise stated. Finally, the structure having the least energy with low RMSD (Root Mean Square Deviation) was used for further studies. In this step, the quality of the initial model was improved. The final structure obtained was analyzed by Ramachandran’s map using PROCHECK (Programs to check the Stereo chemical Quality of Protein Structures) and environment profile using ERRAT graph (Structure Evaluation server). This model was used for the identification of active site and for docking of the substrate with the enzyme.
Active site Identification
Active site of Calsium Sensing Receptor was identified using CASTp server. A new program, CASTp, for automatically locating and measuring protein pockets and cavities, is based on precise computational geometry methods, including alpha shape and discrete flow theory. CASTp identifies and measures pockets and pocket mouth openings, as well as cavities. The program specifies the atoms lining pockets, pocket openings, and buried cavities; the volume and area of pockets and cavities; and the area and circumference of mouth openings.
Docking method
Docking was carried out using GOLD (Genetic Optimization of Ligand Docking) software which is based on genetic algorithm (GA). This method allows as partial flexibility of protein and full flexibility of ligand. The compounds are docked to the active site of the CASR. The interaction of these compounds with the active site residues are thoroughly studied using molecular mechanics calculations. The parameters used for GA were population size (100), selection pressure (1.1), number of operations (10,000), number of island (1) and niche size (2). Operator parameters for crossover, mutation and migration were set to 100, 100 and 10 respectively. Default cutoff values of 3.0 A° (dH-X) for hydrogen bonds and 6.0 A° for vanderwaals were employed. During docking, the default algorithm speed was selected and the ligand binding site in the alpha glucosidase was defined within a 10 A° radius with the centroid as CE atom of PHE220. The number of poses for each inhibitor was set 100, and early termination was allowed if the top three bound conformations of a ligand were within 1.5A° RMSD. After docking, the individual binding poses of each ligand were observed and their interactions with the protein were studied. The best and most energetically favorable conformation of each ligand was selected.
Gold Score fitness function:
Gold Score performs a force field based scoring function and is made up of four components: 1. Protein-ligand hydrogen bond energy (external H-bond); 2. Protein-ligand vander Waals energy (external vdw); 3. Ligand internal vander Waals energy (internal vdw); 4. Ligand intramolecular hydrogen bond energy (internal- H- bond). The external vdw score is multiplied by a factor of 1.375 when the total fitness score is computed. This is an empirical correction to encourage protein-ligand hydrophobic contact. The fitness function has been optimized for the prediction of ligand binding positions.
GoldScore = S (hb_ext) + S (vdw_ext) + S (hb_int) + S (vdw_int)
Where S (hb_ext) is the protein-ligand hydrogen bond score, S (vdw_ext) is the protein-ligand van der Waals score, S (hb_int) is the score from intramolecular hydrogen bond in the ligand and S (vdw_int) is the score from intramolecular strain in the ligand.
Results and Discussion
Homology Modeling of CASR Protein (Q2F3K4)
A high level of sequence identity should guarantee more accurate alignment between the target sequence and template structure. In the results of BLAST search against PDB, only one-reference protein 2E4Uchin A has a high level of sequence identity and the identity of the reference protein with the CASR protein are 31%. Structurally conserved regions (SCRs) for the model and the template were determined by superimposition of the two structures and multiple sequence alignment.

Fig. 1: Blast result with a similar template having 31% identity with CASR.
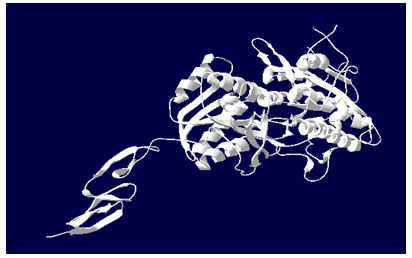
The stable structure of the 2E4Uchin A protein obtained is shown in Fig. 2.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
In the following study, we have chosen 2E4Uchin A as a reference structure for modeling Q2F3K4 domain. Coordinates from the reference protein (2E4Uchin A) to the SCRs, structurally variable regions (SVRs), N-termini and C-termini were assigned to the target sequence based on the satisfaction of spatial restraints.
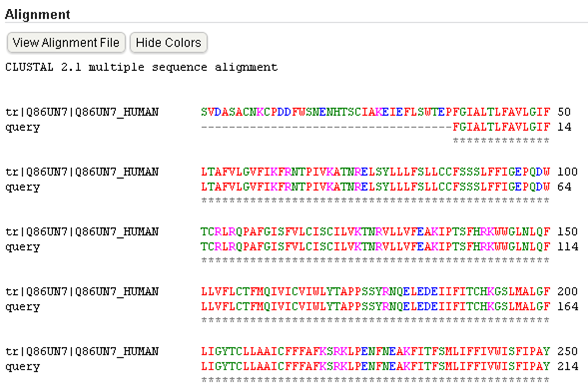

Fig 3: alignment of CASR with template 2E4Uchin A.
In the modeler we will get a 20 PDB out of which we select a least energy. The energy unit will be in kilo joule .All side chains of the model protein were set by rotamers. The final stable structure of the Calcium Sensing Receptor protein obtained is shown in Fig. 4.
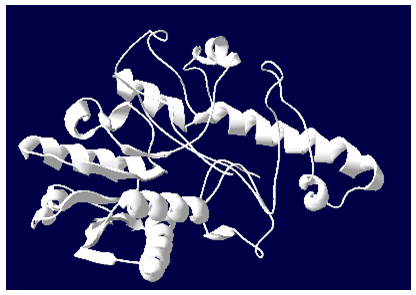
Fig 4: 3D structure of CASR generated by Modeller9V8
By the help of SPDBV it is evident that Calcium Sensing Receptor protein has 10 helices and 8 sheets and it is shown in the Fig. 6.
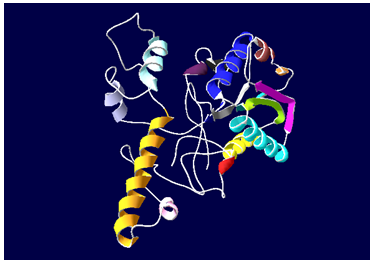
Fig 6: Protein with helices and sheets
The structure having the least energy with low RMSD (Root Mean Square Deviation) which was obtained by the NAMD is in water molecule (TIP3).
Fig 7: CASR with water molecule.
RMSD graph of CASR after energy minimization.
The final structure was further checked by verify 3D graph and the results have been shown in Figure 8. The overall scores indicates acceptable protein environment.
Figure 8: The 3D profiles verified results of Calsium Sensing Receptor model; overall quality score indicates residues are reasonably folded.
Validation of Protien
After the refinement process, validation of the model was carried out using Ramachandran plot calculations computed with the PROCHECK program. The π and ψ distributions of the Ramachandran plots of non-glycine, non-proline residues are summarized in Table 1. Altogether 70.6% of the residues of Calcium Sensing Receptor was in favored and allowed regions. The overall PROCHECK G-factor of Calcium Sensing Receptor (Q2F3K4) and verify3D environment profile were good.
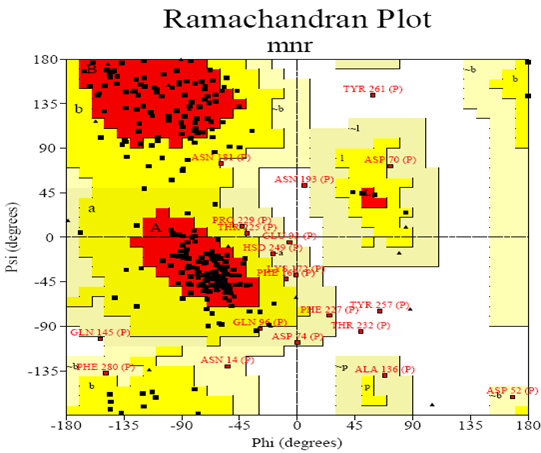
Figure 9: Ramachandran Plot
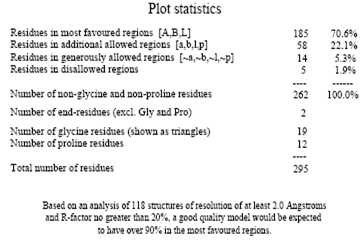
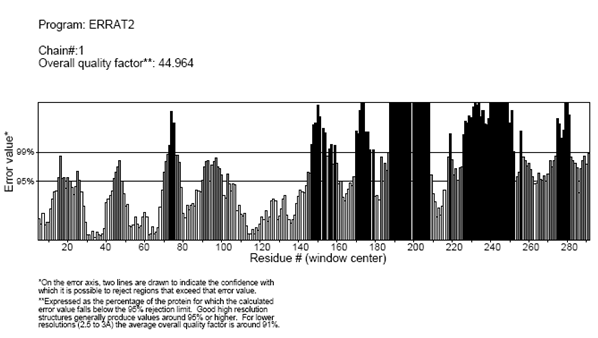
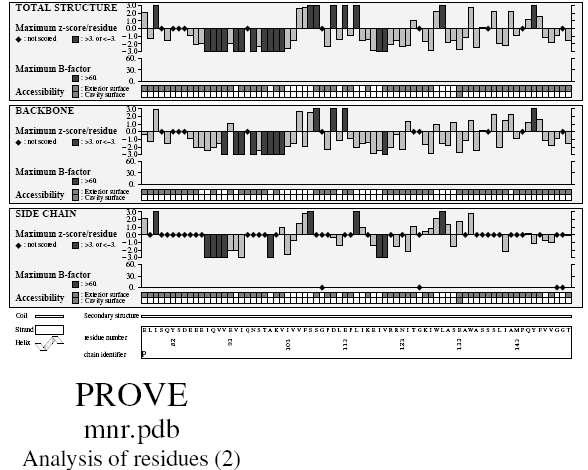
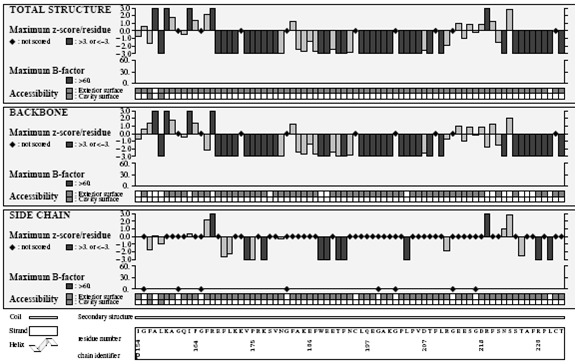
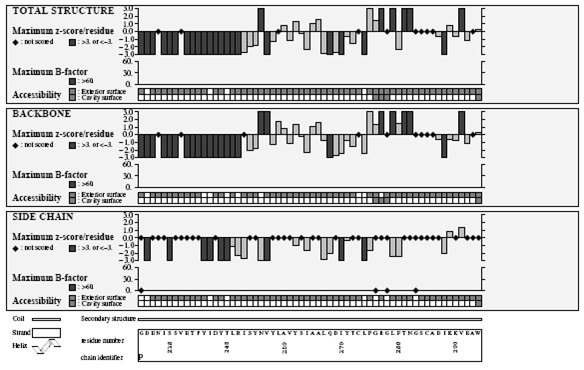
Superimposition of 2E4Uchain A with Calcium Sensing Receptor Protein
The structural superimposition of C trace of template and Signal transducer and activator of transcription 4 is shown in Figure. The weighted root mean square deviation of C α trace between the template and final refined models 1.29Ao. This final refined model was used for the identification of active site and for docking of the substrate with the protein Calcium Sensing Receptor.
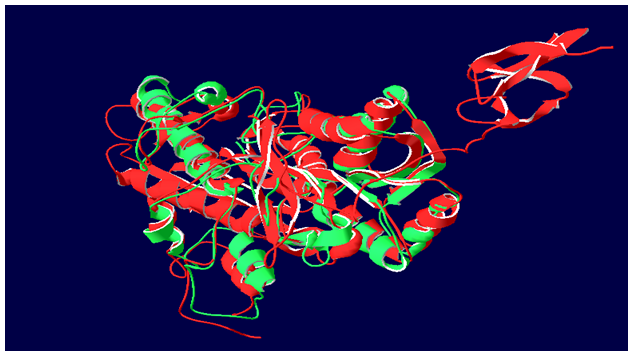
Fig 10: Super imposition of CASR and 2E4Uchin A.
Active site Identification of Calcium Sensing Receptor protein
After the final model was built, the possible binding sites of Calcium Sensing Receptor was searched based on the structural comparison of template and the model build and also with CASTp server and was shown in Figure 5. Since, Calcium Sensing Receptor and the 2E4Uchin A are well conserved in both sequence and structure; their biological function should be identical. Infact from the structure-structure comparison of template, final refined model of Calcium Sensing Receptor protein using SPDBV program and was shown in Figure3. It was found that secondary structures are highly conserved and the residues TYR3, ALA4, ILE23, SER80, GLN81, TYR82, GLY109, PRO110, ASP111, GLU113, ALA131, GLU133, ALA134, TRP135, ALA136, SER137, SER138, SER139, LEU140, ILE141, ALA142, MET143, PRO144, GLN1454, PHE147, VAL150, GLY151, THR153, GLY155, LEU158, ALA168, GLY161, GLN162, ILE163, PHE166, ARG167, PHE169, LEU170, LYS171, VAL173, PRO175, SER178, ASN181, PHE183, ALA184, GLU186, PHE187, TRP188, GLU189, GLU190, THR191, PHE192, ASN193, VAL206, ASP207, THR208, PHE209, LEU210, ARG211, GLY212, GLU214, GLU215, SER216, GLY217, ASP218, PHE220, SER221, LEU279, PHE280, THR281, CYS285, ALA286, ASP287, ILE288.
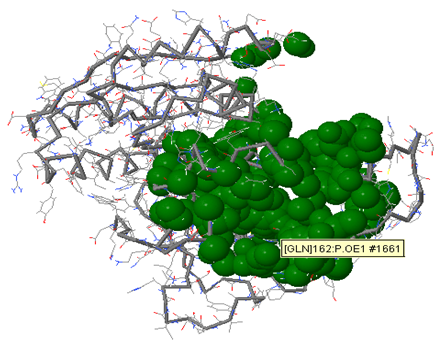
Figure 11: active site of CASR.
The Ligand (inhibitor) molecules used for Docking studies
By modifying ligand molecules, 15 inhibitors are designed and they are listed below:
1. Structure of Strontium Ranelate
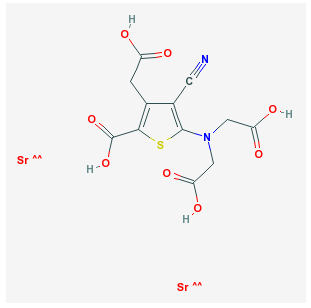
5-[bis(carboxymethyl)amino]-3-(carboxymethyl)-4-cyanothiophene-2-carboxylic acid; strontium
Docking Result Of Strontium Ranelate
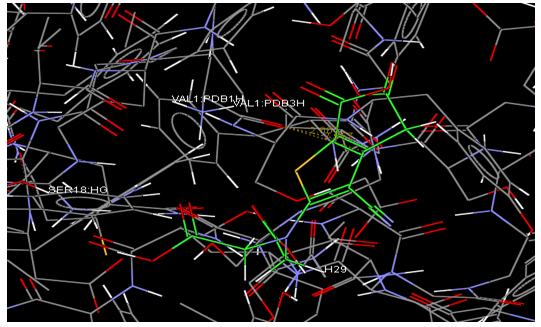
2. Structure of Strontium Ranelate Derivative 1
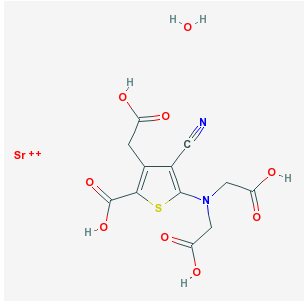
strontium5-[bis(carboxymethyl)amino]-3-(carboxymethyl)-4-cyanothiophene-2-carboxylic acid hydrate
Docking Result Of Strontium Ranelate Derivative 1
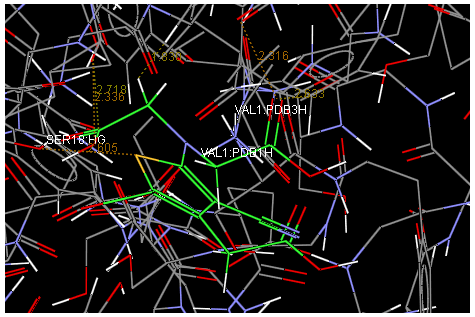
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
3. Structure of Strontium Ranelate Derivative 2
5-[bis(2-hydroxy-2-oxoethyl)amino]-3-(carboxymethyl)-4-cyanothiophene-2-carboxylic acid
Docking Result Of Strontium Ranelate Derivative 2
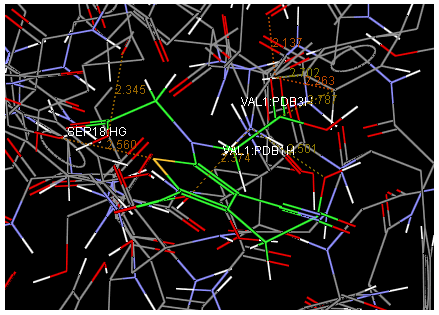
4. Structure of Strontium Ranelate Derivative 3
5-[bis(carboxymethyl)amino]-3-(carboxymethyl)-4-cyanothiophene-2-carboxylic acid
Docking Result Of Strontium Ranelate Derivative 3
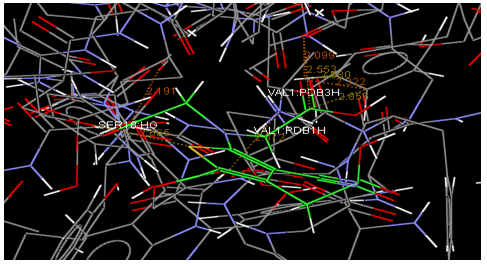
5. Structure of Strontium Ranelate Derivative 4
distrontium5-[bis(2-oxido-2-oxoethyl)amino]-4-cyano-3-(2-oxido-2-oxoethyl)thiophene-2-carboxylate heptahydrate
Docking Result Of Strontium Ranelate Derivative 4
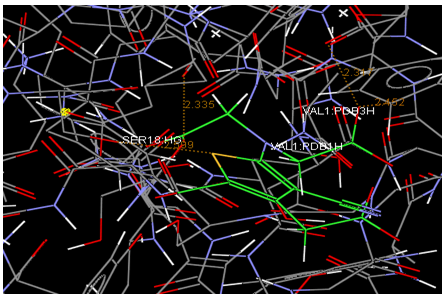
6. StructureofStrontiumRanelateDerivative 5
3-(carboxymethyl)-5-[carboxymethyl(ethyl)amino]-4-cyanothiophene-2-carboxylic acid
Docking Result Of Strontium Ranelate Derivative 5
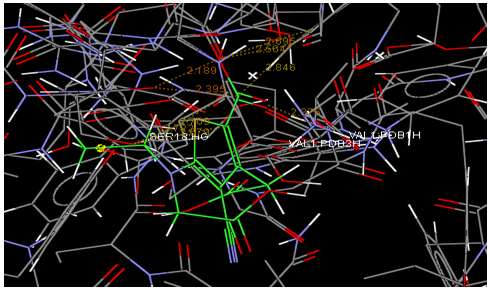
7. Structure of Strontium Ranelate Derivative 6
distrontium5-[bis(2-oxido-2-oxoethyl)amino]-4-cyano-3-(2-oxido-2-oxoethyl)thiophene-2-carboxylate
Docking Result Of Strontium Ranelate Derivative 6
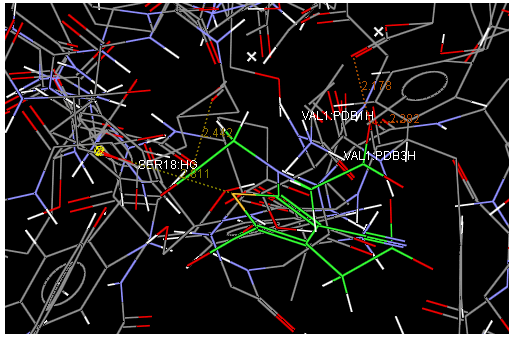
8. Structure of Strontium Ranelate Derivative 7
5-[bis(2-oxido-2-oxoethyl)amino]-4-cyano-3-(2-oxido-2-oxoethyl)thiophene-2-carboxylate
Docking Result Of Strontium Ranelate Derivative 7
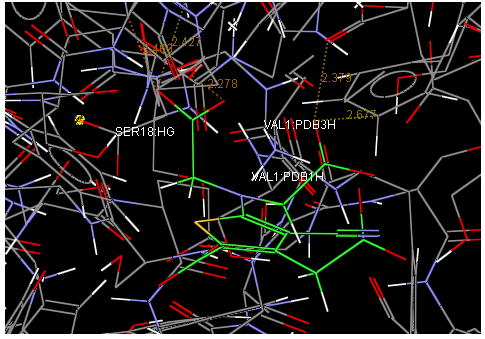
9. Structure of Strontium Ranelate Derivative 8
tetrapotassium5-[bis(2-oxido-2-oxoethyl)amino]-4-cyano-3-(2-oxido-2-oxoethyl)thiophene-2-carboxylate
Docking Result Of Strontium Ranelate Derivative 8
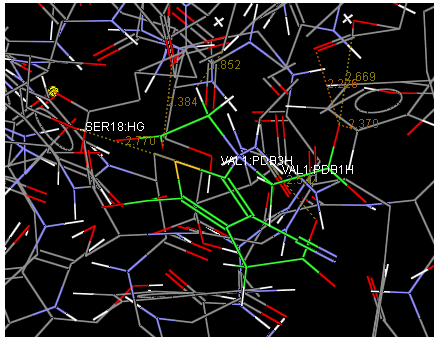
10. Structure of Strontium Ranelate Derivative 9
4-cyano-5-[ethyl-(2-oxido-2-oxoethyl)amino]-3-(2-oxido-2-oxoethyl)thiophene-2-carboxylate
Docking Result Of Strontium Ranelate Derivative 9
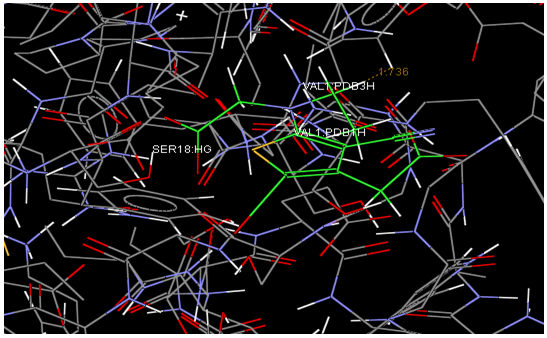
11. Structure of Strontium Ranelate Derivative 10
tetralithium5-[bis(2-oxido-2-oxoethyl)amino]-4-cyano-3-(2-oxido-2-oxoethyl)thiophene-2-carboxylate
Docking Result Of Strontium Ranelate Derivative 10
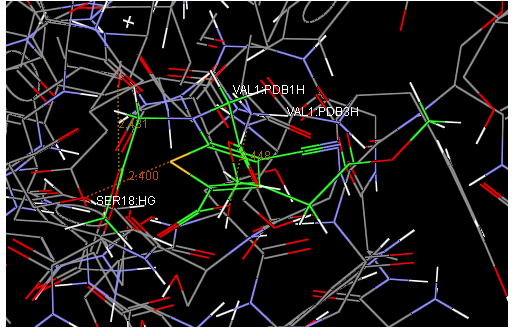
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
12. Structure of Strontium Ranelate Derivative 11
5-[bis(2-methoxy-2-oxoethyl)amino]-4-cyano-3-(2-methoxy-2-oxoethyl)thiophene-2-carboxylic acid
Docking Result Of Strontium Ranelate Derivative 11
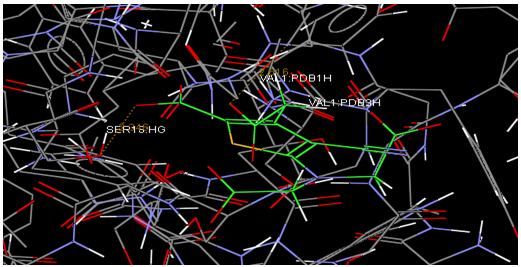
13. Structure of Strontium Ranelate Derivative 12
dicalcium5-[bis(2-oxido-2-oxoethyl)amino]-4-cyano-3-(2-oxido-2-oxoethyl)thiophene-2-carboxylate
Docking Result Of Strontium Ranelate Derivative 12
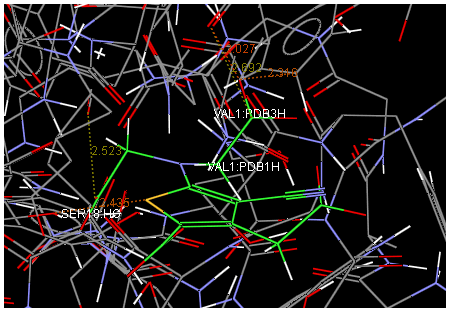
14.Structure of Strontium Ranelate Derivative 13
distrontium 5-[bis(2-oxido-2-oxoethyl)amino]-4-cyano-3-(2-oxido-2-oxoethyl)thiophene-2-carboxylate
Docking Result Of Strontium Ranelate Derivative 13
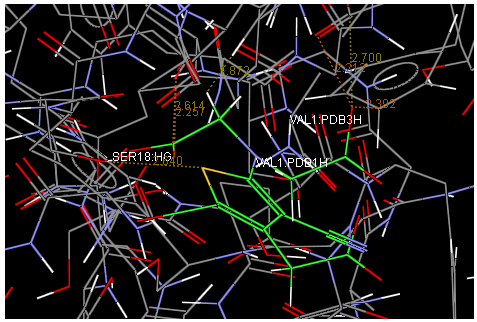
15. Structure of Strontium Ranelate Derivative14
distrontium 5-[bis(2-oxido-2-oxoethyl)amino]-4-cyano-3-(2-oxido-2-oxoethyl)thiophene-2-carboxylate
Docking Result Of Strontium Ranelate Derivative 14
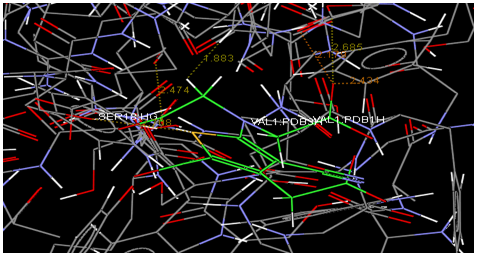
File containing a listing of the fitness of the top-ranked individual for each ligand docked in GOLD.
Mol No Fitness S(hb_ext) S(vdw_ext) S(hb_int) S(int) |
1 32.38 12.98 21.65 0.00 -10.36 |
2 40.20 23.69 21.47 0.00 -13.01 |
3 39.42 11.80 29.35 0.00 -12.74 |
4 37.26 20.40 21.50 0.00 -12.70 |
5 46.68 26.00 21.66 0.00 -9.10 |
6 47.49 25.74 23.56 0.00 -10.65 |
7 44.65 25.00 22.70 0.00 -11.57 |
8 36.14 14.10 24.85 0.00 -12.13 |
9 33.66 13.35 24.21 0.00 -12.98 |
10 35.55 13.07 24.20 0.00 -10.80 |
11 46.36 26.00 23.03 0.00 -11.31 |
12 29.36 7.62 21.47 0.00 -7.77 |
13 43.26 25.88 20.70 0.00 -11.09 |
14 40.98 26.00 19.93 0.00 -12.43 |
15 45.84 25.03 23.57 0.00 -11.59 |
The Chemical Properties these Structures are tabulated as follows:
Table 1
|
S.NO |
Molecular formula |
Formula weight |
Molar refractivitycm3 |
Index of refraction |
Densityg/cm3 |
Polarisability 10-24cm3 |
|
1 |
C12H10N2O8S
|
342.2814 |
72.34±0.4cm3 |
1.695±0.03g/cm3 |
1.81±0.1g/cm3 |
28.68±0.51024cm3 |
|
2 |
C12H10N2O8S
|
342.2814 |
72.34±0.4cm3 |
1.695±0.03g/cm3 |
1.81±0.1g/cm3 |
28.68±0.51024cm3 |
|
3 |
C12H10N2O8S
|
342.2814 |
72.34±0.4cm3 |
1.695±0.03g/cm3 |
1.81±0.1g/cm3 |
28.68±0.51024cm3 |
|
4 |
C12H10N2O8S
|
342.2814 |
72.34±0.4cm3 |
1.695±0.03g/cm3 |
1.81±0.1g/cm3 |
28.68±0.51024cm3 |
|
5 |
C12H6N2O8S
|
338.2518344 |
Not available |
Not available |
Not available |
Not available |
|
6 |
C12H12N2O6S
|
312.29848 |
70.69±0.4cm3 |
1.649±0.03g/cm3 |
1.61±0.1g/cm3 |
28.02±0.51024cm3 |
|
7 |
C12H6N2O8S
|
338.2518344 |
Not available |
Not available |
Not available |
Not available |
|
8 |
C12H6N2O8S
|
338.2518344 |
Not available |
Not available |
Not available |
Not available |
|
9 |
C12H6N2O8S
|
338.2518344 |
Not available |
Not available |
Not available |
Not available |
|
10 |
C12H9N2O6S |
309.2763 |
Not available |
Not available |
Not available |
Not available |
|
11 |
C12H16N2O8S |
384.36114 |
86.85±0.4cm3 |
1.574±0.03g/cm3 |
1.46±0.1g/cm3 |
34.43±0.51024cm3 |
|
12 |
C12H6N2O8S
|
338.2518344 |
Not available |
Not available |
Not available |
Not available |
|
13 |
C12H6N2O8S
|
338.2518344 |
Not available |
Not available |
Not available |
Not available |
|
14 |
C12H6N2O8S
|
338.2518344 |
Not available |
Not available |
Not available |
Not available |
|
15 |
C12H6N2O8S
|
338.2518344 |
Not available |
Not available |
Not available |
Not available |
Docking of inhibitors with the active site of Calsium Sensing Receptor
Docking of the inhibitors with Calsium Sensing Receptor was performed using GOLD 3.0.1, which is based on Genetic algorithimn. This program generates an ensemble of different rigid body orientations (poses) for each compound conformer within the binding pocket and then passes each molecule against a negative image of the binding site. Poses clashing with this ‘bump map’ are eliminated. Poses surviving the bump test are then scored and ranked with a Gaussian shape function. We defined the binding pocket using the ligand-free protein structure and a box enclosing the binding site. This box was defined by extending the size of a cocrystalized ligand by 4 Å. This dimension was considered here appropriate to allow, for instance, compounds larger than the cocrystallized ones to fit into the binding site. One unique pose for each of the best-scored compounds was saved for the subsequent steps. The compounds used for docking was converted in 3D with SILVER. To this set, the substrate corresponding to the modeled protein were added.
Conclusion
In this work, we have constructed a 3D model of CASR Protein, using the MODELLER software and obtained a refined model after energy minimization. The final refined model was further assessed by ERRAT and PROCHECK program, and the results show that this model is reliable. The stable structure of CASR Protein is further used for docking with modified ligand molecules. Docking results indicate that conserved amino-acid residues Calsium Sensing Receptor main play an important role in maintaining a functional conformation and are directly involved in donor substrate binding. The interaction between the domain and the inhibitors proposed in this study are useful for understanding the potential mechanism of domain and the inhibitor binding. As is well known, hydrogen bonds play important role for the structure and function of biological molecules. In this study it was found that, TYR3, ALA4, ILE23, SER80, GLN81, TYR82, GLY109, PRO110, ASP111, GLU113, ALA131, GLU133, ALA134, TRP135, ALA136, SER137, SER138, SER139, LEU140, ILE141, ALA142, MET143, PRO144, GLN1454, PHE147, VAL150, GLY151, THR153, GLY155, LEU158, ALA168, GLY161, GLN162, ILE163, PHE166, ARG167, PHE169, LEU170, LYS171, VAL173, PRO175, SER178, ASN181, PHE183, ALA184, GLU186, PHE187, TRP188, GLU189, GLU190, THR191, PHE192, ASN193, VAL206, ASP207, THR208, PHE209, LEU210, ARG211, GLY212, GLU214, GLU215, SER216, GLY217, ASP218, PHE220, SER221, LEU279, PHE280, THR281, CYS285, ALA286, ASP287, ILE288 are important for strong hydrogen bonding interaction with the inhibitors. To the best of our knowledge VAL1,SER18,SER2,GLU241 are conserved in this domain and may be important for structural integrity or maintaining the hydrophobicity of the inhibitor-binding pocket. The molecule StrontiumRanelate Derivative5 showed best docking results with target protein.
References
1. Brian K Alldredge; Koda-Kimble, Mary Anne; Young, Lloyd Y.; Wayne A Kradjan; B. Joseph Guglielmo (2009). Applied therapeutics: the clinical use of drugs. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. pp. 101–3.
2. Kim DH, Vaccaro AR (2006). "Osteoporotic compression fractures of the spine; current options and considerations for treatment". The spine journal : official journal of the North American Spine Society 6 (5): 479–87.
3. Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ (2007). "Will my patient fall?". JAMA 297 (1): 77–86. doi:10.1001/jama.297.1.77.
4. Waugh, EJ; Lam, MA, Hawker, GA, McGowan, J, Papaioannou, A, Cheung, AM, Hodsman, AB, Leslie, WD, Siminoski, K, Jamal, SA, Perimenopause BMD Guidelines Subcommittee of Osteoporosis, Canada (2009 Jan). "Risk factors for low bone mass in healthy 40-60 year old women: a systematic review of the literature.".Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 20 (1): 1–21.
5. Melton LJ (2003). "Epidemiology worldwide". Endocrinol. Metab. Clin. North Am. 32 (1): 1–13,
6. Raisz L (2005). "Pathogenesis of osteoporosis: concepts, conflicts, and prospects.". J Clin Invest 115 (12): 3318–25.
7. Ojo F, Al Snih S, Ray LA, Raji MA, Markides KS (2007). "History of fractures as predictor of subsequent hip and nonhip fractures among older Mexican Americans". Journal of the National Medical Association 99 (4): 412–8.
8. Poole KE, Compston JE (December 2006). "Osteoporosis and its management". BMJ 333 (7581): 1251–6.
9. Berg KM, Kunins HV, Jackson JL et al. (2008). "Association between alcohol consumption and both osteoporotic fracture and bone density". Am J Med 121 (5): 406–18.
10. Nieves JW (1 May 2005). "Osteoporosis: the role of micronutrients.". Am J Clin Nutr 81 (5): 1232S–1239S.
11. Wong PK, Christie JJ, Wark JD (2007). "The effects of smoking on bone health". Clin. Sci. 113 (5): 233–41.
12. Jasminka Z. Ilich, PhD, RD and Jane E Kerstetter, PhD, RD (2000). "Nutrition in Bone Health Revisited: A Story Beyond Calcium". Journal of the American College of Nutrition 19 (6): 715–737.
13. Abelow BJ, Holford TL and Insogna KL (1992). "Cross-cultural association between dietary animal protein and hip fracture: a hypothesis". Calcified tissue international 50 (1): 14–18.
14. Hegsted M, Schuette SA, Zemel MB and Linkswiler HM (1981)."Urinary calcium and calcium balance in young men as affected by level of protein and phosphorus intake". The Journal of nutrition111 (3): 553–562.
15. Kerstetter JE and Allen LH (1990). "Dietary protein increases urinary calcium". Journal of Nutrition 120 (1): 134–6.
16. Shapses SA, Riedt CS (1 June 2006). "Bone, body weight, and weight reduction: what are the concerns?". J. Nutr. 136 (6): 1453–6.
17. Staessen J, Roels H, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R (Apr 3 1999). "Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group.". Lancet 353(9159): 1140–4.
18. Tucker KL, Morita K, Qiao N, Hannan MT, Cupples LA, Kiel DP (2006). "Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study". Am. J. Clin. Nutr. 84 (4): 936–42.
19. American Academy of Pediatrics Committee on School Health (2004). "Soft drinks in schools". Pediatrics 113 (1 Pt 1): 152–4.doi:10.1542/peds.113.1.152.
20. Simonelli, C et al. (July 2006). "ICSI Health Care Guideline: Diagnosis and Treatment of Osteoporosis, 5th edition" (PDF). Institute for Clinical Systems Improvement. Retrieved 2008-04-08.
21. Kohlmeier, Lynn Kohlmeier (1998)."Osteoporosis - Risk Factors, Screening, and Treatment". Medscape Portals. Retrieved 2008-05-11.
22. Ebeling PR (2008). "Clinical practice. Osteoporosis in men". N Engl J Med 358 (14): 1474–82.
23. Gourlay M, Franceschini N, Sheyn Y (2007). "Prevention and treatment strategies for glucocorticoid-induced osteoporotic fractures". Clin Rheumatol 26 (2): 144–53.
24. Petty SJ, O'Brien TJ, Wark JD (2007). "Anti-epileptic medication and bone health". Osteoporosis international 18 (2): 129–42.
25. Ruiz-Irastorza G, Khamashta MA, Hughes GR (2002). "Heparin and osteoporosis during pregnancy: 2002 update". Lupus 11 (10): 680–82.
26. Gage BF, Birman-Deych E, Radford MJ, Nilasena DS, Binder EF (2006). "Risk of osteoporotic fracture in elderly patients taking warfarin: results from the National Registry of Atrial Fibrillation 2".Arch. Intern. Med. 166 (2): 241–46.
27. Yang YX, Lewis JD, Epstein S, Metz DC (2006). "Long-term proton pump inhibitor therapy and risk of hip fracture". JAMA 296 (24): 2947–53.
28. Murphy CE, Rodgers PT (2007). "Effects of thiazolidinediones on bone loss and fracture". Ann Pharmacother 41 (12): 2014–18.
29. Guglielmi G, Scalzo G. Imaging tools transform diagnosis of osteoporosis. Diagnostic Imaging Europe. 2010;26(May):7-11.
30. Leib ES, Lewiecki EM, Binkley N, Hamdy RC (2004). "Official positions of the International Society for Clinical Densitometry". J Clin Densitom 7 (1): 1799.
31. US Preventive Services Task Force (2002). "Screening for osteoporosis in postmenopausal women: recommendations and rationale". Ann. Intern. Med. 137 (6): 526–28. .
32. Martínez-Aguilà D, Gómez-Vaquero C, Rozadilla A, Romera M, Narváez J, Nolla JM (2007). "Decision rules for selecting women for bone mineral density testing: application in postmenopausal women referred to a bone densitometry unit". J. Rheumatol. 34 (6): 1307–12.
33. Schousboe JT, Taylor BC, Fink HA, et al. (2007). "Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men". JAMA 298 (6): 629–37.
34. Davis S, Oliver A, Goeckeritz B, Sachdeva A (2010). "All about osteoporosis: a comprehensive analysis". Journal of Musculoskeletal Medicine 27 (4): 149–153.
35. Dalsky GP, Stocke KS, Ehsani AA, Slatopolsky E, Lee WC, Birge SJ (1988). "Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women". Ann. Intern. Med. 108(6): 824–28.
36. Sahota, O. (2009). "Reducing the risk of fractures with calcium and vitamin D: The combination is more effective than vitamin D alone". BMJ 339: b5492.
37. DIPART (vitamin D Individual Patient Analysis of Randomized Trials (2010). "Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe". BMJ340: b5463.
38. Rosen, Hillel N. Calcium and vitamin D supplementation in osteoporosis. In: UpToDate, Basow, DS (Ed), UpToDate, Waltham, MA, 2010.
39. Feskanich D, Willett WC, Stampfer MJ, Colditz GA (1996). "Protein consumption and bone fractures in women". Am. J. Epidemiol. 143(5): 472–79.
40. Kerstetter JE, O'Brien KO, Insogna KL (2003). "Dietary protein, calcium metabolism, and skeletal homeostasis revisited". Am. J. Clin. Nutr. 78 (3 Suppl): 584S–592S.
41. Davis S, Sachdeva A, Goeckeritz B, Oliver A (2010). "Approved treatments for osteoporosis and what's in the pipeline". Drug Benefit Trends 22 (4): 121–124.
42. Lyles KW, Colón-Emeric CS, Magaziner JS, et al. (2007). "Zoledronic acid and clinical fractures and mortality after hip fracture". N Engl J Med 357 (18): 1799–809.
43. Purcell, P. Boyd, I (2005). "Bisphosphonates and osteonecrosis of the jaw". Medical Journal of Australia 182 (8): 417–18..
44. Taranta A, Brama M, Teti A, et al. (February 2002). "The selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitro". Bone 30 (2): 368–76.
45. Meunier PJ, Vignot E, Garnero P, et al. (1999). "Treatment of postmenopausal women with osteoporosis or low bone density with raloxifene. Raloxifene Study Group". Osteoporos Int 10 (4): 330–36.
46. Nicholson GC, Moseley JM, Sexton PM, Mendelsohn FA, Martin TJ. Abundant calcitonin receptirs in solated rat osteoclasts. J Clin Invest; 1986; 78:355-360.
47. Okumura S, Mizoguchi T, Sato N, Yamaki M, Kobayashi Y, Yamauchi H, Ozawa H, Udagawa N, Takahashi N (2006). “Coordination of microtubules and the actin cytoskeleton is important in osteoclast function, but calcitonin disrupts sealing zones without affecting microtubule networks“. Bone. 39 (4): 684-693.
48. El-Khoury GY, Moore TE, Albright JP, Huang HK, Martin RK. Sodium Fluoride Treatment of Osteoporosis: Radiologic Findings. AJR. 1982;139:39-43
49. Meunier PJ, Roux C, Seeman E, et al. (2004). "The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis". N. Engl. J. Med. 350 (5): 459–68.
50. O'Donnell S, Cranney A, Wells GA, Adachi JD, Reginster JY (2006). Cranney, Ann. ed. "Strontium ranelate for preventing and treating postmenopausal osteoporosis". Cochrane database of systematic reviews (Online) (4): CD005326.
51. Reginster JY, Seeman E, De Vernejoul MC, et al. (2005). "Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: treatment of peripheral osteoporosis (TROPOS) study.". J Clin Endorinol Metab90 (5): 2816–22.
52. Blake GM, Fogelman I (2007). "The correction of BMD measurements for bone strontium content". J Clin Densitom 10 (3): 259–65.
53. Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A (2007). "Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis". Lancet 370 (9588): 657–66.
54. Prince RL, Devine A, Dhaliwal SS, Dick IM (2006). "Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women". Arch. Intern. Med. 166 (8): 869–75.
55. Bolland MJ, Barber PA, Doughty RN, et al. (2008). "Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial". BMJ 336 (7638): 262.
56. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B (2005). "Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials".JAMA 293 (18): 2257–64.
57. Jackson RD, LaCroix AZ, Gass M, et al. (2006). "Calcium plus vitamin D supplementation and the risk of fractures". N. Engl. J. Med. 354 (7): 669–83.
58. Bonaiuti D, Shea B, Iovine R, et al. (2002). Bonaiuti, Donatella. ed. "Exercise for preventing and treating osteoporosis in postmenopausal women". Cochrane database of systematic reviews (Online) (3): CD000333.
59. Houtkooper, LB, Stanford, VA, Metcalfe, LL, Lohman, TG, and Going, SB (2007). Preventing osteoporosis the Bone Estrogen Strength Training way. journal=ACSM's Health & Fitness Journal.11. pp. 21–27.
60. Cheng S, Sipilä S, Taaffe DR, Puolakka J, Suominen H (2002). "Change in bone mass distribution induced by hormone replacement therapy and high-impact physical exercise in post-menopausal women". Bone 31 (1): 126–35.
61. Chien MY, Wu YT, Hsu AT, Yang RS, Lai JS (2000). "Efficacy of a 24-week aerobic exercise program for osteopenic postmenopausal women". Calcif. Tissue Int. 67 (6): 443–48.
62. Iwamoto J, Takeda T, Ichimura S (2001). "Effect of exercise training and detraining on bone mineral density in postmenopausal women with osteoporosis". Journal of orthopaedic science : official journal of the Japanese Orthopaedic Association 6 (2): 128–32.
63. Kemmler W, Engelke K, Weineck J, Hensen J, Kalender WA (2003). "The Erlangen Fitness Osteoporosis Prevention Study: a controlled exercise trial in early postmenopausal women with low bone density-first-year results". Archives of physical medicine and rehabilitation 84 (5): 673–82.
64. Kerr D, Morton A, Dick I, Prince R (1996). "Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent". J. Bone Miner. Res. 11 (2): 218–25.
65. Villareal DT, Binder EF, Yarasheski KE, et al. (2003). "Effects of exercise training added to ongoing hormone replacement therapy on bone mineral density in frail elderly women". J Am Geriatr Soc51 (7): 985–90.
66. Sinaki M, Brey RH, Hughes CA, Larson DR, Kaufman KR (2005). "Significant reduction in risk of falls and back pain in osteoporotic-kyphotic women through a Spinal Proprioceptive Extension Exercise Dynamic (SPEED) program". Mayo Clin Proc 80 (7): 849–55.
67. Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD (2007)."Low bone mineral density and fracture burden in postmenopausal women". CMAJ 177 (6): 575–80.
68. Hannan EL, Magaziner J, Wang JJ, et al. (2001). "Mortality and locomotion 6 months after hospitalization for hip fracture: risk factors and risk-adjusted hospital outcomes". JAMA 285 (21): 2736–42.
69. Brenneman SK, Barrett-Connor E, Sajjan S, Markson LE, Siris ES (2006). "Impact of recent fracture on health-related quality of life in postmenopausal women". J. Bone Miner. Res. 21 (6): 809–16.
70. Riggs, B.L.; Melton, Lj 3.r.d. (2005). "The worldwide problem of osteoporosis: insights afforded by epidemiology.". Bone 17 (5 Suppl): 505S–511S.
71. Cauley JA, Hochberg MC, Lui LY et al. (2007). "Long-term Risk of Incident Vertebral Fractures". JAMA 298 (23): 2761–67.
72. Albright F, Bloomberg E, Smith PH (1940). "Postmenopausal osteoporosis". Trans. Assoc. Am. Physicians. 55: 298–305.
73. Patlak M (2001). "Bone builders: the discoveries behind preventing and treating osteoporosis". FASEB J. 15 (10): 1677E–E.
Declaration
Author hereby declare that the work presented in this dissertation has been originally carried out by him under the supervision of and overall Guidance of D. JAYASIMHA RAYALU, Managing Director, Akshaya Biological Corporation, Himayath Nagar, Hyderabad.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to Pharmatutor Job Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









