ABOUT AUTHOR
DR. PRANJAL BORDOLOI
VP – PHARMACOLGY & COD
VEEDA CLINICAL RESEARCH PVT LTD.
info@veedacr.com
Therapeutic innovation in cancer treatment has always been in focus. Based on WHO data, worldwide, there were 14.1 million new cancer cases, 8.2 million cancer deaths, and 32.6 million people living with cancer within 5 years of diagnosis by year 2012. Top 5 most frequent cancers in world (ranked by number of new cases) are breast, prostate, lung, colorectal, and cervical cancers per 2012 WHO data (International Agency for Research on Cancer, WHO). For countries like India, the top 5 most frequent cancers (ranked by number of new cases) are breast, cervical, oral cavity, lung and colorectal cancers (International Agency for Research on Cancer, WHO). Reported age-adjusted incidence rates for cancer are still quite low in the demographically young country. Little more than 1 million new cases of cancer are diagnosed every year in India. An estimated 600 000–700 000 deaths in India were caused by cancer in 2012. In age-standardized terms this is close to the mortality burden seen in high-income countries. Such figures are somewhat indicative of low rates of early-stage detection and poor treatment outcomes (Mallath MK, et al. 2014).
[adsense:336x280:8701650588]
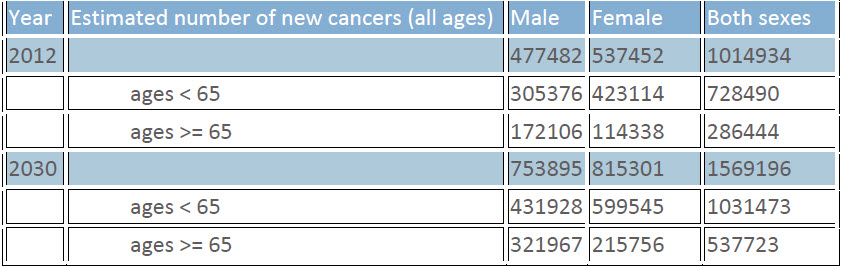
The cancer burden continues to increase due to aging population and increasing adoption of behavioral patterns mainly smoking, sedentary lifestyle and dietary changes with inclination towards junk foods, in economically developing countries. By year 2030, countries like India may see more than 50 % rises in incidence of cancers, compared to data available for the year 2012 (GLOBOCAN 2012 data) (Ferlay J et al., 2012).
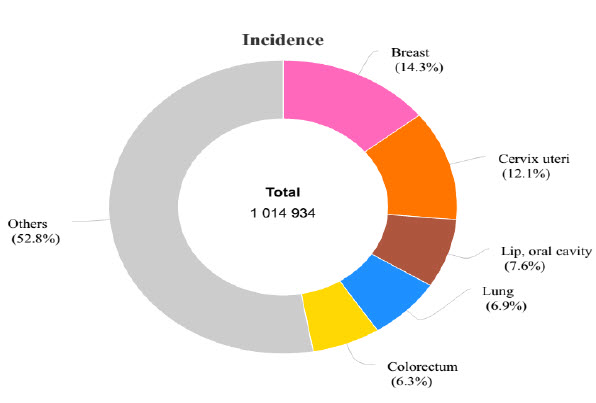
Figure 1: Incidence of different cancer types - India (Source: GLOBOCAN 2012)
Table 1: Estimated number of new cases of cancers in India by 2030 (compared to 2012 data from GLOBOCAN 2012)
Oncology Drug Development Trend
The global cancer therapeutics market should reach $172.6 billion by 2022 at a compound annual growth rate (CAGR) of 7.4%, from 2017 to 2022.vi In past few years, cancer research has seen increased emphasis on personalized medicine and targeted therapies. Due to continuous advances and shift in treatment modalities towards targeted anti-cancer drugs, there has been steady rise in the novel therapeutic approaches and clinical trials in oncology. Many different targeted therapies have been approved for use in cancer treatment. These therapies include hormone therapies, signal transduction inhibitors, gene expression modulators, apoptosis inducers, angiogenesis inhibitors, immunotherapies, and toxin delivery molecules (National Cancer Institute. Targeted Cancer Therapies. Accessed Apr 2018). Targeted therapeutic approaches like, immuno-oncology PD-1 and PD-L1 inhibitors, have seen rapid uptake with patients in developed regions since their first approval, as the number of drugs and the tumor types for which they are approved has increased significantly. Clinical research has become an important element to address the global cancer burden in improving the lives of patients.
More than 50% more cancer drugs enter in late-stage development as compared to last decade. Trends of large number of trials using biomarkers to stratify patients is indicative of pointing to even more personalized cancer treatments in the future. The immunotherapy trials are being conducted across more than 30 different tumor types, indicating the broad application of this new approach to cancer treatment.
With growing cost of drug development, pharma and biotech companies are looking for low-cost avenues for their trials and looking for bigger patient pool for treatment naïve patients. With evolving regulatory scenario with respect to clinical trials in some of the developing countries like India provide an opportunity to these companies to extend the clinical trial programs to these regions. Also the rising incidence of cancer in India, the demand for quality products for treating the disease has steeply risen.
Oncology Clinical Trials in India: Advantages
Regulators in country like India with having increased cancer burden, have started considering clinical trials as a treatment alternative to the patient pools who cannot have access to the treatment modalities. With dialogues between the regulators, subject matter experts and stakeholders from industry, the regulatory environment has significantly improved in last few years. Early lengthy process of approval has been streamlined and now become simple and structured. Other potential advantage of conducting clinical trials include established commercial presence, large patient access pool, low cost for run of the trial as compared to developed regions. Trained English speaking investigators, clinical trial staff, acceptable medical practice which supports conduct of clinical trial as per protocol designed in western countries, broad adoption of ICH-GCP guidelines and rationalization of regulatory structures.
Overcoming the challenges
Regulatory environment having rational approach with uncomplicated approval processes is the basis for sound clinical trial review process. This provides platform for thorough scientific review of clinical trials and the trial initiation can be done in reasonable time which is beneficial for not only applicants but also for the patients at large. In country like India, in past few years, regulatory environment has significantly improved after extensive involvement of regulators with professional and industry groups. They have successfully overcome some operational challenges faced by the clinical trial industry. Several measures by the regulators offer constructive and favorable outcome for Indian clinical trial industry. Clinical trial professionals with sound academic credentials and skillsets are key to robust clinical trial planning. Site selection with required patient pool, dedicated trial staff and proper infrastructure is also a vital aspect for reliable and scientific output of any clinical trial. Investigational product supply management, biological sample handling and transportation and accredited laboratories evaluating endpoints are also the crucial aspects in successful clinical trial conduct.
Discussion
With increasing cancer burden throughout the world and considerable research spending in targeted cancer therapies, clinical trials undertaken in this therapeutic area has increased significantly. Countries like India with its improved regulatory environment, large patient pool with unmet medical needs, robust clinical trial professional network, investigators and infrastructure offers comprehensive opportunities to pharma and biotech companies to conduct their clinical trials in India in a cost-effective and prompt manner. Additional investment in capacity building and infrastructure in tier 2, tier 3 cities will contribute to better the governance and management of clinical research, helping to further advance patient safety and protection.
REFERENCES
1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11.Lyon, France: International Agency for Research on Cancer, 2013
2. International Agency for Research on Cancer, WHO. Population Factsheets. Accessed Jul 2018. Available from: http://gco.iarc.fr/today/fact-sheets-populations?population=900&sex=0
3. Mallath MK, Taylor DG, Badwe RA et al. The growing burden of cancer in India: epidemiology and social context. Lancet Oncol. 2014 May;15(6):e205-12
4. National Cancer Institute. Targeted Cancer Therapies. Accessed Apr 2018. Available from: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet
5. Report: Therapies for Resistant and Recurrent Metastatic Cancer, November 2017. Report ID: 5200531
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT editor-in-chief@pharmatutor.org
Subscribe to Pharmatutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









