About Authors:
HH SAPARIA*, M BAIDYA, AR MAHESH
Krupanidhi College of Pharmacy, Chikkabellandur, Carmelaram Post,
Varthur Hobli, Banglore-560035, Karnataka, India.
*hiteshsapariya29@gmail.com
Abstract:
Current advancement in drug discovery technology and search for novel chemical diversity have intensified the efforts for exploring lead from “Ayurveda” the traditional system of medicine in India. Lantana camara, Family: Verbenaceae has been important coniferous plant in ayurvedic and indigenous medicinal systems. The Clinical trials and animal research support the use of Lantana camara for anti-spasmodic, carminative, anti-tumour, anti-inflammatory, anti-malarial, anti-ulcer genic, treatment of emotional stress and trauma, anti-microbial, insecticidal, fungicidal, asthma. Major biochemical constituents of Lantana camara were identified as alkaloids/flavonoids, saponins/tannins, germacrene-A, B and D, triterpenes like lantadenes-A, B, C, D, valencene (principle constituent) and γ-gurjunene, verbascoside, martynoside.This paper includes the evidence-basedoverview of pharmacological and phytochemical properties of the aerial parts of Lantana camara.
[adsense:336x280:8701650588]
Reference Id: PHARMATUTOR-ART-1460
Introduction:
The plant Lantana camara linn. Family- Verbanaceae is commonly known as wild sage or red sage and lantana weed. A large scrambling evergreen, strong smelling shrub with stout recurred prickles, leaves were opposite, often rugose, scabrid on both sides, flowers were small, normally orange but often white to dark red, in heads which are prominently capitates; bracts conspicuous and persistent. Fruits were small, 5 mm diameter, greenish?blue, blackish, drupaceous and shining with two nutlets. Seeds were germinated easily throughout central and south India in most dry stony hills and black [1].
The different parts of plant extract were useful in various diseases like diaphoretic, tonic, antispasmodic, wounds, ulcers, swelling, carminative, tumours and rheumatism, anti-spasmodic, carminative, anti-tumour[1], anti-inflammatory[2], anti-malarial[3], anti-ulcerogenic, treatment of emotional stress and trauma[4], anti-microbial, nematididal, insecticidal, fungicidal[5], influenza, asthma[6],antidote to snake venom, eczema[7], gastrointestinal disorders anti-nociceptive, anti-pyretic, inhibitor of acetyl cholinesterase[8], abortificient[9],anthelmintic, diaphoretic, febrifuge, carminative, rheumatism[10], to make cheap furniture, skin diseases[11], adulticidal activity, larvicidal, biological control[12] .
Major biochemical constituents of Lantana camara were identified as triterpenes like lantadenes-A,B,C,D[1], alkaloids, flavonoids[4], saponins, tannins[13], germacrene-A,B and D, valencene (main compound) and γ- gurjunene[14].
[adsense:468x15:2204050025]
Geographical distribution:
Lantana camaraplant is found mostly in the South India, in Tamilnadu (Kuppandapalayam)[1], in America[5], in Africa[6], mostly native to subtropical and tropical America, but a few taxa were indigenous to tropical Asia and Africa[14], and also found in Himachal Pradesh, Jammu-Kashmir, and Uttar Pradesh[15].
100 genera and 2000 species of Verbanaceae family, Lantana is a genus of 150 species that were very popular as popular ornamental garden plant[6]. Among of 150 species of Lantana camara, some species are L.achyrantifolia Desf., L.indica Roxb., L.montevidensis Briq., L.salvifolia Jacq., L.orangemene, L.trifolia, L.tiliaefolia Cham, L.viburnoides Vahl. Butterflies are said to love Lantana camara flowers when bloom, young flowers are in yellow colour and old are in red colour [13].
The plant is serious weed in the hilly terrecins and also in the plains of the country weeds were known to be inhibit growth of neighbouring vegetation due to release of phytotoxins[15].
Phytochemistry:
Lantana camara, family-Verbanaceae contain major phytochemical constituents were[14],
1) Mono and Sesquiterpenes:They contain bisabolene derivatives with traces of monoterpenes and β-curcumene(1.5%), E-nuciferal, z-nuciferol(3.9%), (-)ar-curcumene-15-al(5.6%), γ-curcumene(8%), ar-curcumene(9.7%), (-)epi-βbisabolol(10%), (-)γ-curcumene-15-al(14.9%).
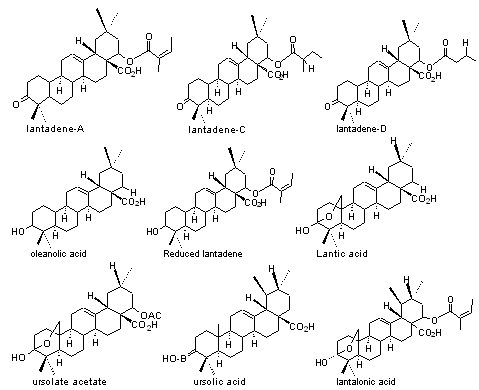
2) Triterpenes:Lantadene-A,B,C,D, Pentacyclic triterpenes like oleanolic acid, ursolate acetate, ursolic acid, reduced lantadene, lantalonic acid, lantic acid.
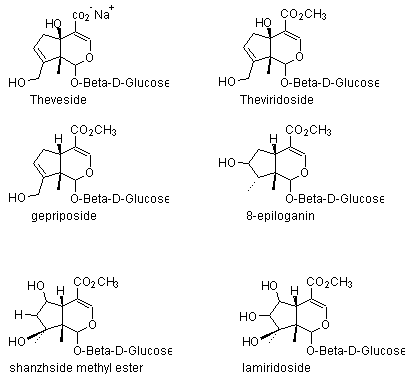
3) Iridoid glycosides: Theveside, theviridoside, gepriposide, 8-epiloganin, shanzhside methyl ester, lamiridoside.
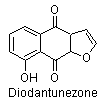
4) Furanonaphthaloquinones: diodantunezone.
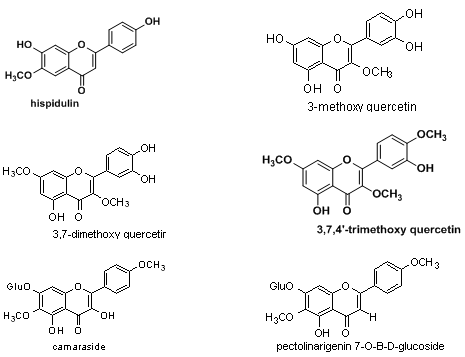
5) Flavonoids:3-methoxy quercetin, 3,7-dimethoxy quercetrin, 3,7,4’-trimethoxy quercetin, camaraside from Lantana camara and fromL.camara var aculeata-pectolinarigenin-7-O-β-D-glucoside.
6) Phenylethanoid glycosides:verbascoside, martynoside, derhamnosylverbascoside, isomumioside, calceolarioside E.
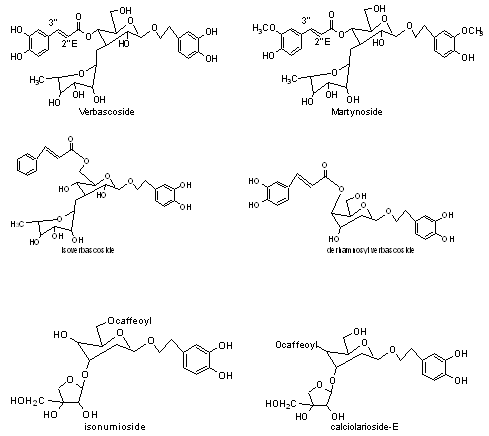
7) Miscellaneous: contain β-sitosterol, campesterol, stigmasterol has been isolated from plant and stems contain high yield of stigmasterol and six oligosaccharides like ajugose, stachyose, verbascotetraose, verbascose, lantanose A, lantanose B has been isolated from roots of Lantana camara.
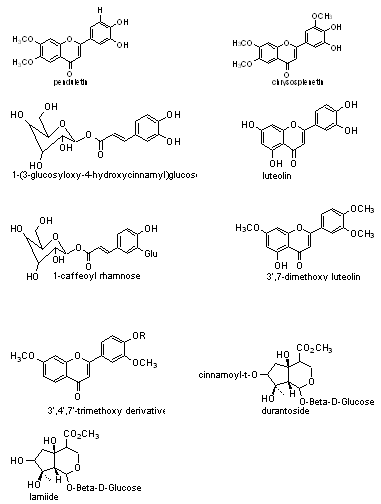
8) Phytochemistry of other Lantana species: The flavones like penduletin, chrysosplenetin has been isolated from aerial parts of Lantana achyranthifolia desf. Whereas from extract of L.hydria contain 1-(3-glucosyloxy-4-hydroxycinnamyl) glucose, from flowers-1-caffeoylrhamnose and monoterpene glucoside ester from L.lilacia Desf. And from L.montevidensis - luteolin and its 3’,7-dimethoxy and 3’,4’,7’- trimethoxy derivatives and iridoid glycosides from roots of L.viburnoids like lamiide, durantoside has been isolated.
Pharmacological and biological activity:
The Lantana camara plant has been used to treat a wide variety of disorders. It was found to use in folk remedies for cancers and tumours. In Central and South America, the leaves were made into a poultice to treat sores, chicken pox and measles.
In Asian countries, leaves were used to treat cuts, rheumatism, ulcers and as a vermifuge. Decoctions were applied externally for leprosy and scabies. L.camara also used to treat cold, rheumatisms, asthma and high blood pressure with preparations from the plant.
A tea prepared from the Lantana camara leaves and flowers was taken against fever, influenza and stomach-ache. In Ghana, infusion of the whole plant was used for bronchitis and the powdered root in milk was given to children for stomach-ache.
From the leaves, an alkaloid fraction which lowered blood pressure, accelerated deep respiration and caused shivering in dogs was isolated. It was suggested that it may be useful in reducing fevers, and as a treatment of asthma and hypertension.
It has been claimed that a steroid, lancamarone from the leaves exhibited cardio tonic properties and lantamine, an alkaloid from the stem bark and roots showed antipyretic and antispasmodic properties, but the validity of these claims has not been confirmed [14].
The roots of Lantana camara rich in olenolic acid. A hepatoprotective triterpenoid and translactone containing triterpene from lantana leaves showed thrombin inhibitory activity. A pentacyclic triterpenoid showed attention for drug research for anti-cancer, anti-microbial, AIDS, anti-inflammatory activity and contain Lantadene - A,B,C, oleanolic acid(major).
A flavonoid in L.trifolia leaves contain umuhengerin as anti-microbial and triterpenoid like ursolic acid used for anti-inflammatory activity and in stems leaves, roots contain oleanolic acid, ursolic acid. L.filiaefolia given orally for anti-hyperlipidemic, anti-tumour activity and contain verbascoside (a protein kinase-c inhibitor) for anti-tumour activity and methanolic extract inhibit human thrombin and 20-pentacyclic triterpenoids like oleanane, ursane, lupine has been obtained[15].
Anthelmintic activity:
L.camaraethanolic extract (10, 50, 100mg/ml concentration) investigated for its anthelmintic activity against pheretima posthuma due to presence of tannins which bind to proteins in the GIT of host animal or glycoprotein on the cuticle of parasite and cause death [10].
Wound healing activity:
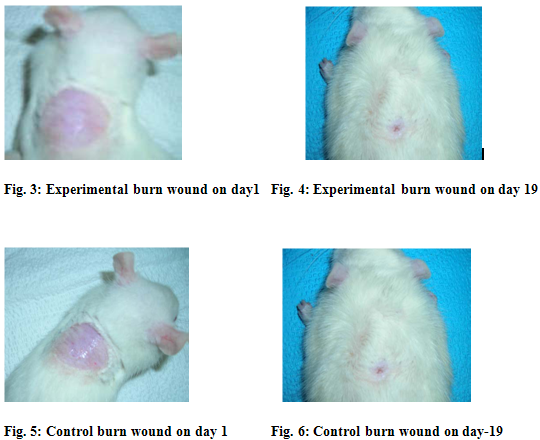
The leaf extract of Lantana camara has been showed antiseptic, anti-leprosy activity. The ethanol extract of L.camara increased the rate of wound contraction by 87 % in burn wound when compared to controls (82%). The slight reduction in the wound area might be due to the antimicrobial effect of the leaf extract against Staphylococcus aureus, Klebsiella andEscherichia coli. By comparing between 1st and 19th day significant wound healing activity showed in Sprague dowley rats[2] (figure-3,4,5,6). The hydroalcoholic extract and fresh juice of L.camara leaves have flavoured wound contraction (kurian 1995) [9].
Larvicidal activity:
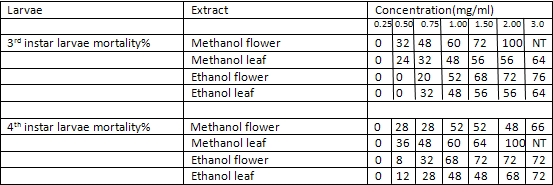
The ethanolic and methanolic extract of Lantana camara leaves and flowers showed a good larvicidal activity against mosquito species like Aedes aegypti and culex quinquefassciatus. It was also showed the maximum mortality with 0.1mg/ml concentration for 24 hours[3] (Table-1 and 2).
A wide range of chemical compounds has been isolated from L.camara was found to be used as larvicidal activity [12].
Aqueous and acetone extract of L.indica leaves were governed for 24 to 96 hours to the fresh water snails lymnaea acuminata and larvae of culex quinquefasciatus. LC50 decreases from 7.32 mg/l (24 hrs) to 1.02 mg/l (96 hrs) against L.acuminata and 10.99mg/l (24 hrs) to 5.18mg/l (96 hrs) against C.quinquifasciatus, so L.indica was used as natural pesticide for vector control [16].
Senthil Nathan et.al 2006 were studied L.camara for their larvicidal action, which act against fourth instar of culex quinquefasciatus (kalayanasundaram and dos 1985) [17].
Essential oil of L.camara has highest adulticidal activity against anopheles fluviatilis followed by anopheles stephensi, anopheles culicifacies(malaria), culex quinque fasciatus(filariasis), aedes aegypti(dengue, chikungunya) and for longer period due to low volatile nature of oil [18].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Antiulcerogenic activity:
The methanol extract of leaves (MELC)showed significant decrease in the gastric lesions in aspirin, ethanol and cold resistant strain induce rats.It has been showed that anti?ulcer activity of extract was dose dependent manner. MELC decreases volume of gastric juice, total acidity, free acidity and increases pH significantly (P<0.001) in aspirin induced gastric ulcer. Pre?treatment with the extract (200 and 400mg/kg) showed ulcer protective effect in aspirin induced (63.31%, 71.02% protection), ethanol induced (85.79%, 93.09% protection) and coldrestraint stress induced (46.86%, 63.90% protection) ulcer models [4].
L.camara extract significantly reduced ulcer index, total acidity, and significantly increase gastric PH of aspirin+pylorus-ligation induced ulcerogenesis and ethanol induced gastric ulcer models. The inhibition zone in diameter of extract against H.pyroli was 20 mm [19].
Nematicidal activity:
The complete extraction of Lantana camara in various solvent was done and it was showed that organic extract caused significantly mortality (nematicidal potential) in meloidogyne javanica juveniles in vitro where as aqueous and methanolic extracts were demonstrated greater inhibition compared to ethyl acetate or n-hexane extracts which indicated that the active principles were polar in nature and contain lantanoside, linoroside, lantanone, camarinic acid[5].
L.camara shows nematicidal activity against root-knot nematode meloidogyne incognita (begun et.al 2008) [20].
Extracts of the leaves, twigs, stems and roots of Lantana camara were screened for activity in the brine shrimp lethality test (BST). The active fractions yielded known oleanonic acid, lantadene A and oleanolic acid, which were very toxic to brine shrimp larvae and not lethal to Spodoptera littoralis Biosduval, Clavigralla tomentosicollis Stal. And Aphis craccivora Koch when tested at 5000µg/ml, lantadene-A however, suppressed the fecundity of C. tomentosicollis at this concentration [21].
Topical activity against dermatophilosis:
Ointment prepared with ethanolic extract of Lantana camara leaves, Senna alata, Mitracarpus scaber was used for topical treatment of chronic crusty or acute lesions of dermatophilosis and also hair grows on the treated areas, for more 3 years-no recurrence has been seen[22].
Anti-inflammatory activity:
The Extracts of Lantana camara and its fractions was investigated using stabilization of red blood cell membrane lysing technique. The whole plant extract gives positive reaction for saponins, flavonoids, ethanol extract gives positive reaction for tannins while ethyl acetate fraction for flavonoids and butanol fraction for saponins. The percentage membrane stability compared with Ibuprofen, Indomethacin and Ethanol protects the erythrocyte membrane while ethyl acetate shows highest protection against induced lyses[23].
Insecticidal activity:
Insecticidal activity of Lantana camara and repellant property after 21 days(82.7-90% insect mortality), the mean lethal exposure times(LT50) to achieve 50% mortality varied from 5-6 days (7.5-10%w/w) to seven to eight days (2.5-5%w/w)[24].
Synthetic mosquito repellants shows side effects so natural insecticide L.camara leaves methanol extract investigated for highest repellent activity against anopheles stepheni with an average 2.63 mosquitos landings whereas hexane extract shows 3.17 mosquito landings were found in Ibadan, Nigeria [25].
Lantana camara and coconut oil extract used against aedes aegypti (94% protection for 2 hr), aedes albopictus (50% protection for 4 hr) (Dua et.al 1996) [26].
L.camara hexane extract shows insecticidal activity against bean weevil (93%) and maize weevil (73.3%) after 24 hr treatment [27]. L.camara has insecticidal activity against stored grain pest, vegetable crops pest[18].
Anti-bacterial activity:
Antibacterial activities of Lantana camara leaf and yellow, lavender, red, white flower ethyl acetate, acetone, chloroform extract prepared and evaluated by agar well diffusion method.
Theflower extracts possess strong antibacterialactivities more than the corresponding leaf extracts.
However, onlyethyl acetate extracts showed to be most effective against all of the bacteriaexcept Salmonella aureus. Acetone and chloroformextracts did not show any significantinhibitory effects against the bacteria.All four types of L.camara flower extractsdisplayed almost similar antibacterial activitieswith zone of inhibition value ranging from 10-21 mm.
L.camara yellow andwhite flowers extracts showed the highestinhibitory effects against Bacillus subtilis with a zone of inhibition of 21 and20 mm respectively. Leaf extracts comparedto the flower extracts, displayed lessinhibitory effects against all the bacteriatested with a relatively smaller zone ofinhibition area ranging from 9-15 mm.Escherichia coli was found to be the most sensitive bacteria to allL. camara flowers and leaf extracts. P.aeruginosa and Bacillussubtilis was also found to be highly susceptible to all L.camara flower and leafextracts[28].
L.camaraproduced minimum inhibitory concentrations (MICs) and minimum bactericidal concentrations (MBCs) of37.5 mg/ml against both Salmonella aureus, pseudomonas aeruginosa and shows appreciable results against mycobacterial tuberculosis[29].
L.camara used against microbial diseases because ethanolic extract shows activity against bacillus substilis, pseudomonas aeruginosa than aqueous extract and no activity against salmonella aureus[30].
H37Rv and TMC-331 are internationally used as standard mycobacterium tuberculosis (MTB) strains which were rifampicin sensitive and rifampicin resistance, respectively. The values for rifampicin were 1.0 µg/ml for both H37Rv and wild strain but rifampicin hardly showed any activity on TMC-331.
The minimum bactericidal concentration (MBC) value for the methanol extract of L. camara was 30µg/ml for the H37Rv, and 20µg/ml for both the TMC-331 and wild strains of M. Tuberculosis where as MBC for rifampicin was 2.0µg/ml for both H37Rv and the wild strain. L. camara extracts showed an advantage over rifampicin by being highly active against the rifampicin-resistant strain of mycobacterium tuberculosis (MTB)[31].
Methanol, petroleum ether, water, chloroform extracts of L.camara roots and stems shows antibacterial activity against gram positive Salmonella aureus, staphylococcus aureus and gram negative E.coli, pseudomonas aeruginosa [32].
L.camarahaving more antibacterial activity against E.coli, bacillus substlis, klebsiella, pneumonia, Salmonella aureus than croton spariflorus and antigonon leptopus[33]. Water extract of L.camara active against fungal pathogens and bacteria [34].
Methanol extract is better than chloroform extract for anti-microbial activity from different parts of plant [35].L.camara shows minimum inhibitory concentration (MIB) against p.vulgeris and p.cholerae[36]. Aromatic oil of L. camara shows bactericidal activity against E.coli, Salmonella.aureus and has bacteriostatic activity against bacillus species [37].
Antiurolithiatic and anti-oxidant activity:
Ethanolic extract of Lantana camara leaves showed antiurolithiatic activity against 0.75% v/v ethylene glycol and 2% w/v ammonium chloride induced calcium oxalate urolithiasis and for antioxidant activity against hyperoxaluria induced oxidative stress in male albino rats. After treatment with the extract, a significant reduction in the deposition of calcium, oxalate and also urinary excretion of calcium, oxalate and creatinine was observed, indicating its antiurolithiatic effect. The administration of extract showed decreased the extent of lipid peroxidation and hence enhanced the levels of antioxidant enzymes in the kidneys of urolithic rats, reflecting its antioxidant efficacy against hyperoxaluria induced renal oxidative stress[38].
Anti-Leukaemia activity:
In 1991, Herbert J.m.et al reported that verbascoside isolated from L.camara possess anti-tumour activity in vitro due to inhibition of protein kinase c. Shashi B.M.et al finding Lantana’s extracts have anti-tumour activity. Lantadenes and related triterpenoid inhibited Epstein - Barr virus activation, making hope to build anti-tumour promoters by few changes in chemical structure (the substituents on the carboxylic acid through an ester bond).
Some triterpenoids like 22-beta-acetoxylantic acid and 22-beta-dimethyl acryloxyloxy lantanoic acid has been reported to anti-mutagenic. Study on lantadenes and their esters by Sharma.et.al reveled the importance of the groups attached to C-22 and C-17 in relation to anti-tumour activity.
Games-howel statistical test showed that leaf and root extract of L.camara had similar anti-protective activity (p>0.1, n=3) after 24 hrs and 72 hr that was constrastable with carboplatin (p<0.05,n=3). L.camara leaf extract has about 1/11.5 times as anti-leukaemia activity as carboplatin after 72 hrs (leaf extract IC50, 394.41+99.73 μg/ml; carboplatin IC50, 34.83+3.60 μg/ml) and root extract around 1/9.5 times as effect as this drug at same interval (root extract IC50, 328.36+53.08 μg/ml). L camara root and leaf extract had roughly equal anti-proliferative activity on leukaemia jurkat cell line by the mechanism of apoptosis induction, but this activity was about 1/10 of carboplatin potency, a reference anti-cancer drug[39].
L. camarashows anti-proliferative activity in percent of living cell in laryngeal cancer cell line (55.98+0.74) and percentage of living cells in lung cancer cell line(25.8+0.19)[40].
Anti-filarial activity:
L.camarahas anti-filarial activity against human lymphatic filarid brugia malayi (80%) and rodent filarid acanthocheiconema viteae (95%) maintained in rodent rats. Crude extract at 1g/kg for 5 days by oral route killed 43.05% of adult brugia malayi and sterilized 76% of surviving female worms in the rodent model mastomys coucha.
A 34.5% adulticidal activity along with sterilization of 66% of female worms could be in chloroform fraction. Oleanonic acid and oleanolic acid from hexane and chloroform fractions showed LC100 at 31.25 and 62.5 μg/ml respectively on b.malayi in vitro. This is first ever reported on the anti-filarial efficacy of L.camara[41].
Anti-motility activity:
Effect of L.camara on neostigmine induced gastrointestinal transit in mice investigated. neostigmine used as premotility agent, intestinal motility accessed by charcoal meal test, the intestinal transit with L.camara methanolic extract(LCME) at a dose of 500mg/kg was 26.46% where at 1mg/kg completely inhibited the transit of charcoal in normal mice. The intestinal transit in the neostigmine pre-treated groups was 24 and 11 at the same dose respectively. Plant extract at 125 to 250mg/kg doses were administered intraperitonially shows significant reduction in faecal output compared with castor oil treated mice, at higher dose(500-1000mg/kg) the fecal output completely stopped, so L.camara was used in secretory and functional diarrhoea caused by castor oil[42].
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Toxicity studies on Lantana camara:
Research on the plant Lantana camara showed that after ingestion of lantana foliage by grazing animals causes intrahepatic cholestasis and ruminants like cattle, buffalo, sheep and non-ruminants like horse, rabbits, guinea pigs susceptible to the Lantana camara. Lantana poisoning in livestock has been reported in India, U.S.A, Australia, Brazil, Indonesia, and Africa.
Incidence of Lantana poisoning reported during transportation of animal’s lantana free regions to lantana infested areas or on leaving the animals for grazing in lantana infested 8 localities after some period of stall feeding. Incidences of Poisioning in buffalo (Kangra valley, H.P.), sheep, goats (in Rampur bushier) and after eating lantana foliage, the animals suffer from constipation and anorexia. After 24-48 hours animals get jaundice, photosensitive, subsequently eye-lids become hairless.
Lantadene-A,B were toxic to guinea pig, sheep. Lantadene-A were most toxic principle in the plant, Lantadene-C was showed hepatotoxicity in guinea pigs, after absorption of toxins were transported to liver in portal blood. Toxins resemble cholesterol and absorption of cholesterol is facilitated by esterification with cholesterol esterase. Biotransformation and disposition investigated in guinea pigs and lantana is not detected in liver, bile, blood, urine, gall bladder.
The metabolites of Lantana camara like lantadene-A, B were showed in G.I.T. and in faeces. L.camara poisoning causes photosensitisation due to retension of phylloerythrin which is normally excreted in bile and jaundice due to accumulation of bilirubin as a result of inhibition of bile secretion. Administration of activated charcoal at the rate of 500g in 4 liters of electrolyte to sheep and 2.5 kg in 20 liters of electrolyte given to cattle for the treatment[15].
Cytotoxicity test on Vero line cell showed that the leaf extract of Lantana camara at concentration up to 500 mg/ml inhibited the growth of cells 2.5 times less than that done by triton 100.1% and started to decline at elevated concentration while female mice loose body weight, liver damage after single dose of leaf extract in acute toxicity test and male lost organs-heart and kidney[43].
It was the first weed for biological control at the turn of century and was the serious weed in the plantation crops like coffee, palm oil, coconuts, cotton, it invades in Australia, East Africa, Fiji, India, South Africa, Zambia, Zimbabwe. Leaves and seeds contain triterpenoids were toxic to sheep, cattle shows photosensitivity and death due to poisoning[44].
L.camarawas potencially toxic and shows photosensitivity, hepatotoxicity, nephrotoxicity, intestinal haemorrhage, dermatitis[9]. L.camara is a major invader of forest, pastuer, watelands throughout India (Dobhal 2010), ability to grow under wide range of climatic conditions (dat et.al), alleo chemicals released by roots in the soil inhibiting the growth of neighbouring plant, significant loss (28.4%) of species richness and 63% loss of basal area ofvegetation was recorded in the invaded localities compared to non-invaded oneson nayar region in Garhwal Himalayas (Uttarkhand) India [45].
L.camarais top in terms of highest impacting invasive plant species (batin off and batler 2003) and one of the world’s 100 worst invasive alien species (GISP 2003) and depletion of tree population in the Vidhyan tropical dry deciduous forest in India [46].
Effect of poisoning by the L.camara cause ruminal stasis and marked decreased in fore stomach motility 4-6 hour after dosing with plant and continued to be depressed throughout the course due to inhibiting impulses arising from damaged liver [47].
Accidental poisoning with L.camara cause liver damage and accumulation of phylloerythrin in the blood which sensitize animal skin to UV light, marked inflammation in eyes and sexual orifices, in cattle, buffalo, guinea pig-jaundice, rise in glutamic oxaloacetic transaminase, elevated hepatic and renal xanthine oxidase activity[48].
As the lantana camara is a most noxious plant, toxicity depends up on three phases- (1) The release and absorption of toxins in the G.I.T. (2) The hepatic phase resulting in cholestasis, hyperbilirubinemia, hypererythremia. (3) Tissue phase where in injury results from the accumulation of bilirubin and phylloerythrin[49].
Alleochemicals from lantana plant were lantadene-A,B and umbelliferone, methyl coumarin. In recent reports on bilirubin clearance effect of Chinese herbal tea in zhi huang or its active ingredient 6,7-dimethyl esculetin in jaundice were exiting investigation on possible ameliorative effects of lantana intoxicified animals[50].
Triterpines-lantadene -A,B produces jaundice, ruminal stasis, hepatic and ruminal cholestasis. the decresed ruminal motility causes retained toxic material in rumen and absorption maintains the disease, treatment by large quantity of activated charcoal in rumen with multiple of electrolyte solution to stimulate ruminal motility and rehydrate the animal [51]
L.camara var. aculeatagiven in dose of 6g/kg body weight to guinea pig produces cholestasis, after euthanized the animal L.camara could not be in blood and urine samples and find in lower G.I.T and feces. [52]
Single dose (1-3 mg/kg) of L.camara produces liver injury after injected i.v. in sheep due to triterpinene acid-lantadene-Aand chatracterised by transient rises in serum enzymes with or without hyperbilirubinemia. In higher dose produce hepatic necrosis and in lower doses produce cholestatic syndrome for several days[53].increase in hepatic and renal xanthine oxidase activity and causes rise in glutamicoxaloacetic transaminase acitivity, obstructive jaundice, photosensitisation when ingested L.camara in cattle, sheep, buffalo, guinea pig[54].
Bentonite and activated charcoal given by stomach tube as a 5g/kg in calves, five of six calves in each of the groups given bentonite and activated charcoal recovered while 5 of 6 calves in the control groups died, so bentonite have cheap alternative to activated charcoal in cattle[55].
500g of activated charcoal in 4 liter electrolyte given orally can protect sheep against intoxification, mortality was higher in intoxified sheep given only electrolyte and untreated sheep, six cows with 2-2.5 g activated charcoal in 20-30 liter electrolyte solution given by stomach tube recovered naturally occurring lantana poisoning [56].
Dried alcoholic extract of L.camara leaves on oral administration to albino rats causes photo dermatitis [57], pressure changes occurring in the jejunam,dueodenum,caecum and spiral colon of sheep were recorded due to L.camara in intestinal motility, L.camara didn’t affect responses of duodenum to pentagastrin given i.v. or hydrochloric acid,so L.camara does not cause intestinal paralysis [58].
The polar and apolar extract of L.camara given by i.p route (1.5, 3.0, 5.0 g/kg) to mice , both extract shows death after 2 days shows both extract have some common principles but only apolar extract presented dose dependent increased lethality[59]. Effect of l.camara on general reproductive performance and teratology in rats has been investigated, the data showed interfered in the frequency of fetal skeleton anomalies from dams treated with extract and induced embryotoxicity indicated by post implantation loss without any sign of maternal toxicity[60].
The l.camara extract did not interfere with all over weight or internal organ weight but interfere with sperm count, daily sperm production and sperm morphology in dose dependent manner[61].
Vaccination as a possible means of preventing lantana poisoning-the toxic triterpene acids lantadene-A,B were isolated and conjugated bovine serum albumin or haemocyanin. The conjugates were emulsified with complete freund’s adjacent and injected in to sheep and cattle .vaccinated animals produced antibodies against the toxic compounds cholestasis was less severe in vaccinated sheep challenged with a toxic dose of lantana. The results indicated a mild protective effect of vaccination against the hepatotoxic effect of toxins [62].
Acknowledgement:
The authors acknowledge Professor Dr Suresh Nagpal, Chairman, Krupanidhi Institutions, for providing necessary facilities like various journals, books and digital library to carry out the review work in an effective manner.
Conclusion:
From the above review we can conclude that the plant Lantanacamarawhichis having a wide range of medicinal value due to their variety of chemical constituents can be further investigated on toxicological and other parameters to obtain a valuable marketed product. The specific part of the plant has to identified for their pharmacological use and the different pharmaceutical approach has to applied to formulate suitable dosage form.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE
Figures:

Tables:
TABLE 1:
Mortality percentage of early 3rd and 4th instar larvae of Aedes aegypti (25 larvae) subjected for 24 hr to different concentration of methanol and ethanol extracts of Lantana camara. Values are mean of three replicates Concentration
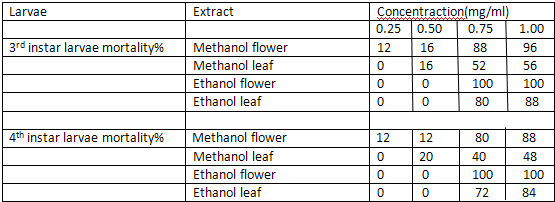
TABLE 2:
Mortality percentage of early 3rd and 4th instar larvae of culex quinque fasciatus(25 larvae) subjected for 24 hr to different concentration of methanol and ethanol extracts of Lantana camara. Values are mean of three replicates Concentration
References:
1. Venkatachalam T, Kishorkumar V, Selvikalai P, Avinash M, Senthilkumar N. Phytochemical and preliminary phytochemical studies on the Lantana camara fruits. Int J Pharm and Pharm Sci 2011;3(1):52-54.
2. Nayak BS, Raju SS, Ramsubhag A. Investigation of wound healing activity of Lantana camara in Sprague Dawley Rats using a burn wound model. Int J Appl Res Nat Pro 2008;1(1):15-19.
3. Satishkumar M, Maneemegalai S. Evaluation of larvicidal effect of Lantana camara against mosquito species aedes aegypti and culex quinquefasciatus. Advan. Biol. Res 2008;2(3-4):39-43.
4. Thamotharan G, Sekhar G, Ganesh T. Anti-ulcerogenic effects of Lantana camara leaves on in vivo test models in Rats. Asian J Pharm Clin Res 2010;3(3):57-60.
5. Ali NI Siddiqui A, Zaki MJ, Shokat SS. Nematicidal potential of Lantana camara against meloidogyne javanica in mungbean. Nematol.Medit 2001;29:99-102.
6. Arecelio VC, Sydney GL, Erlanio OS, Fabiola FG, Adriana RC, Josegalberto MC. Effect of collection time in essential oil composition of Lantana camara (verbanaceae) growing in Brazil northeastern. Rec. Nat. Pro. 2010;4(1):31-37.
7. Vanaprasad B, Prasanth KK, Chandrasekhar N, Somasekhar P. Anti-fungal activity of selected plant extracts against phytopathogenic fungus aspergillus niger F2723. Ind J. Science. Technol 2009;2(11):63-66.
8. Adalgisa IM et.al. Osmotic and morphological effects on red blood cell membrane: action of an aqueous extract of L.camara. Brazilian Journal of Pharmacognosy 2008;18(1):42-46.
9. Shahid P, Rajinder R, PK Verma, Pankaj NK. Medicinal plants and their role in wound healing. Vetscan 2008;3(1).
10. Jitendra P, GS Kumar, Deviprasad SP, Shamimqureshi MD. Phytochemical and anthelmintic evaluation of Lantana camara(L) var aculeate leaves against pheretima posthuma. Journal of Global Trends in Pharmaceutical Sciences 2011;2(1):11-20.
11. Chittaranjan D, SK acharya. Effect of fiber content on abrasive wear of Lantana camara fiber reinforced polymer matrix composite. Indian Journal of Engineering & Materials Sciences 2010;17:219-223.
12. Jitendra P, GS Kumar, Shahimqureshi MD, Bharatkumar D, Ashokumar K. Phytochemicals and pharmacological activities of Lantana camara Linn. Research Journal of Pharmacognosy and Pharmacodynamics 2010;2(6):418-422.
13. Mariyajancyrani P, Kumarravel S, Kannan PS. GC-MS analysis of Lantana camara. Indian Journal of Pharmaceutical Research & Development 2009;2(11):63-66.
14. Ghisalberti EL. A review article on Lantana camara (Verbenaceae). Fitoterapia 2000;71:467-486.
15. Om PS. Overview of Research on the hepatotoxic plant-Lantana camara. www.hillagric.ernet.in.
16. Manisha S, VK S hrivastava, Ajay S .Molluscicidal and mosquito larvicidal efficacy of Lantana camara indica Roxb. Leaf extracts. Natural Product Radiance 2007;6(2):122-126.
17. Nashima R, Noreddine S, Abdelouahed A. Larvicidal activity of a neem tree extract (azadiracha tin) against mosquito larvae in the republic of Algeria. Jordan Journal of Biological Sciences 2009;2(1):15-21.
18. VK Oua, AC Pandey, AP Dash. Adulticidal activity of essencial oil of Lantana camara leaves against mosquitoes. Indian J Med Res 2010;131:434-439.
19. Sathish R, Bhusan V, Natarajan K. Antiulcerogenic activity of Lantana camara leaves on gastric and duodenal ulcers in experimental rats. Journal of Ethanopharmacognosy 2011;134(1):195-197.
20. Mohammad R et. al . An ethanomedical pharmacological and phytochemical review of some bignoniaceae family plants and a description of biognoniaceae plants in folk medicinal uses in Bangladesh. Adv. in Nat. Appl. Sci. 2010;4(3):236-253.
21. Majekodunmi OF, Labaran S, Stephen KS, Yoshio T. Larvicidal activity of extracts and triterpenoids from Lantana camara. Pharmaceutical Biology 2002;40(8):564-567.
22. Emmanuel NA, Akakpo JA, Moudachirou M, Leclercq JQ. Treatment of bovine dermatophilosis with senna alata, Lantana camara and mitracarpus scaber leaf extracts. Journal of Ethanopharmacology 2003;86:167-171.
23. Ooyedapo O, Akinpelu BA, Akinwanmi KF, Adeyinka MO, Sipeolu FO. Red blood cell membrane stabilizing potentials of exracts of Lantana camara and its fractions. Adv. in Nat. Appl. Sci. 2010;2(4):46-51.
24. Ogendo JO, Walker DJ, Belmain SR, Deng AL. Comparision of toxic and repellant effects of Lantana camara with tephysosia vogeii hook and a synthetic pesticide against sito philus zeamais motschulsky in stored maize grain. Insect Sci. Applic. 2003;23(2):127-135.
25. Eganyomi A, Gbadamosi IT, Osiname KO. Comparitive effectiveness of ethanobotanical mosquito repellants used in Ibadan, Nigeria. J. Appl. Biosci. 2010; 36:2383-2388.
26. Plant origin and repellants, bioenvironmental stratergy for malaria control, Page no.171-174.
27. Extracts of three plants against Insecticidal action of hexane extracts of three plants against bean weevil, Callosobruchus maculatus (F.) and maize weevil, Sitophilus zeamais motsch. Journal of Ecology and the Natural Environment 2011;3(1):23-28.
28. Deepak G, Silviya S, Kishwar HK. Biochemical composition and antibacterial activities of Lantana camara plant with yellow, lavender, red, white flowers. EurAsia J BioSciences 2009;3:69-77.
29. Richard MM, Pauloo, John AO, Claude K, Joseph NO, Efficacy of 13 medicinal plants used by indigenous communities around lake Victoria, Kenya against tuberculosis, diarrhoea causing bacteria and candida albicans. International Journal of Pharmacy and Technology 2010;2(3):771-791.
30. Guptaraj N, Viswas K, Manoj P, surendra PS, gupta alka. Antibacterial activities of ethanolic extracts of plants used in folk medicine. International Journal of Research in Ayurveda & Pharmacy 2010;1(2):529-535.
31. Claude K, Paul Waako, Moses Joloba, Otwa oydek. The antimycobacterial activity of Lantana camara plant traditionally used in south-western Uganda. Afr Health Sci 2010; 4(1):40-45.
32. Mary Kensa V. Studies on phytochemical screening and anti-bacterial activities of Lantana camara Linn. Plant Sciences feed 2011;1(5):74-79.
33. Bhuvneshwari S, Arvind R, Kaviyarasan V, Sekharbabu H, Kalivanan KA. comparitive study on antibacterial activity of common weeds. International Journal of Pharma and Biosciences 201;677-683.
34. Rajesh D, Amita G, Mandal TG, Deshdeepak S, Vivek B,Gaurav AM. Lavekar GS. Anti-microbial activity of some Indian medicinal plants. Afr.J.Trad.Cam 2007;4(3):313-318
35. Naem quasar, Basir ahmed C, Altaf D, Abdul M, Rahan Z. Phytochemical study of aerial parts of Lantana camara for the pharmacological active compounds. J App Pharm 2009 ;1(1):219-226.
36. Baretto FS et.al. Anti-bacterial activity of Lantana camara Linn. And lantana montevidensis brig extracts from carri-ceara, Brazil. J Young Pharmacists 2010;2:142-44.
37. Smaranika P, Bonita P. A study of Lantana Camara linn. Aromatic oil as an anti-bacterial agent. Int RJ Pharm Sci 2010;01(01):0032.
38. Mayee R, Thosar A. Evaluation of Lantana camara Linn. (verbanaceae) for Antiurolithiatic and Antioxidant activities in rats. Int. J Pharm clin. Res. 2011;3(1):10-14.
39. Mahdi P, Sashidharan S, Rameshwar NJ, Ramnathan S. Anti-leukemia activity of methanolic extracts of Lantana camara. Phcog Res 2009;1:274-279.
40. Jaobe gomes de Melo et.al. Antiproliferative activity,antioxidant capacity and tannin content in plants of semi-rigid Northeastern Brazil. Molecules 2010;15:8534-8542.
41. Namita M, Mithilesh S, Kanwal R, Anil B, Sudhir S and Shaila M. Chemical constituents and antifilarial activity of L.camara against human lymphatic filarid acantechelone ma viteae maintained in rodent rats. Bio Medical and Life Sciences. 100(3):439-448.
42. Sagar L, Sehgal R, Ojha S. Evaluation of antimotility effect of L.camara Var.aculeata constituents on neostigmine induced gastrointestinal transit in mice. BMC Complement Altern Med 2005;5(1):18.
43. Badakhshan MP, Sreenivasan S. Cytotoxicity and oral acute toxicity studies of Lantana camara leaf extract. Molecules 2011; 16: 3663-3674.
44. Sarah ET, Carol AE. A century of classical biological control of Lantana camara:can pathogen make a significant difference?. Neel R Spencer(ed.)2000;97-100.
45. Praveenkumar D, Ravinderkumar K, Daizy rani B. Impact of L.camara L. Invasion on ripanian vegetation of Nayan region in Garwhal Himalayas(Uttarkhand,India). J. Ecol. Nat. Environ. 2001;3(1):11-12.
46. Gyan PS, Raghubansi AS. Effect of Lantana camara l. Cover on local depletion of tree population in the vidhyan topical dry delicious forest in India. Applied Ecology and Environmental Research;5(1):109-121.
47. Christopher S. Sweeney MC, Michael AP. The mechanism of ruminal stasis in Lantana camara poisoned sheep. Quaterly Journal of experimental physiology 1983;68:301-313.
48. Ross garden cooper. Accidental poisoning from L.camara(cherry pie)Hay fed to ostriches. Turk. J. Vet. Anim. Sci. 2007;31(3):213-214.
49. Sharma OP, Makkar HP, Davra RK. A review of the noxious plant lantana camara. Toxicol 1988;26(11):975-87.
50. Sharma OP, Sharma S, Pattabhi V, Mahato SB, Shama PD. A review of the hepatotoxic plant lantana camara. Crit Rev Toxicol 2007;37(4):313-52.
51. Pass MA. Current ideas on the pathology and treatment of lantana poisoning in ruminants. Aust Vet J 1986;63(6):169-171.
52. Sharma S, Sharma OP, Singh B, Bhat TK. Biotransformation of lantadenes,the pentacyclic triterpenoid hepatotoxins of lantana plant, in guinea pig. Toxicon 2000;38(9):1191-202.
53. Pass MA, Seawight AA, Lamberton JA, Heath JJ. Lantadene A toxicity in sheep: A model of cholestasis. Pathology 1979;11(1):89-94.
54. Sharma OP, Makkar HP, Dawra RK, Neggi SS. A review of the toxicity of L.camara in animals.Clin toxicol 1981;18(9):1077-911.
55. MC Kenzie RA. Bentonite as therapy for lantana camara poisoning of cattle. Aust Vet J; 1991;68(4):146-8.
56. Pass MA, Stewart C. Administration of activared charcoal for the treatment of lantana poisoning of sheep and cattle. J Appl Toxicol 1989;4(5):266-9.
57. Akhter MH, Mathur M, Bhide NK. Skin and liver toxicity in experimental lantana camara poisoning in albino rats. Indian J Physiol Pharmacol 1990;34(1):13-6.
58. Pass MA, TJ Heath. The effect of lantana camara on intestinal motility in sheep.
59. Bevilacaua AH, Safferdini IB, Romoff P, Lago JH, Bernardi MM. Toxicity of poar and apolar l.camara crude extract in mice. Res Vet Sci 2011;90(1):106-115.
60. Mello FB, Jacobus D, Carvalho k, Mello JR. Effects of lantana camara(verbanaceae) on general reproductive performance and teratology in rats. Toxicon 2005;45(4):459-66.
61. De Mello FB, Jacobus D, Carvalho KC, De Mello JR. Effects of L.camara(verbanaceae) on rat fertility. Vet Hum Toxicol 2003;45(1)20-3.
62. Stewart C, Lamberton JA, Fairclough RJ, Pass MA.vaccination as a possible means of prevents lantana poisoning. Australian Veternory journal 1988;65(11):349-352.
NOW YOU CAN ALSO PUBLISH YOUR ARTICLE ONLINE.
SUBMIT YOUR ARTICLE/PROJECT AT articles@pharmatutor.org
Subscribe to PharmaTutor Alerts by Email
FIND OUT MORE ARTICLES AT OUR DATABASE









